Korean J Hepatobiliary Pancreat Surg.
2013 Feb;17(1):21-33. 10.14701/kjhbps.2013.17.1.21.
Therapeutic potentials occurring during the early differentiation process of mesenchymal stem cells in a rats model with thioacetamide-induced liver fibrosis
- Affiliations
-
- 1Department of Surgery, Gachon University Gil Hospital, Incheon, Korea.
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 3Department of Anatomy and Cell Biology, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2243165
- DOI: http://doi.org/10.14701/kjhbps.2013.17.1.21
Abstract
- BACKGROUNDS/AIMS
Mesenchymal stem cells (MSCs) have the capacity to differentiate into hepatocytes, The purpose of this study is to investigate the MSCs' differentiation process and therapeutic potentials by comparing isolated MSCs with HGF-treated MSCs in rat's model with thiacetamide (TAA)-induced cirrhosis.
METHODS
Male Sprague-Dawley (SD) rats, weighing 100-150 g were used in this study. To induce liver fibrosis, recipient rats were taken with 0.04% thioacetamide (TAA) in the drinking water (400 mg TAA/L) for 8 weeks. The rats underlying liver cirrhosis were divided into 3 groups according to the transplanted materials, compared to normal saline as control (I) and isolated MSCs (II) HGF-treated MSCs.
RESULTS
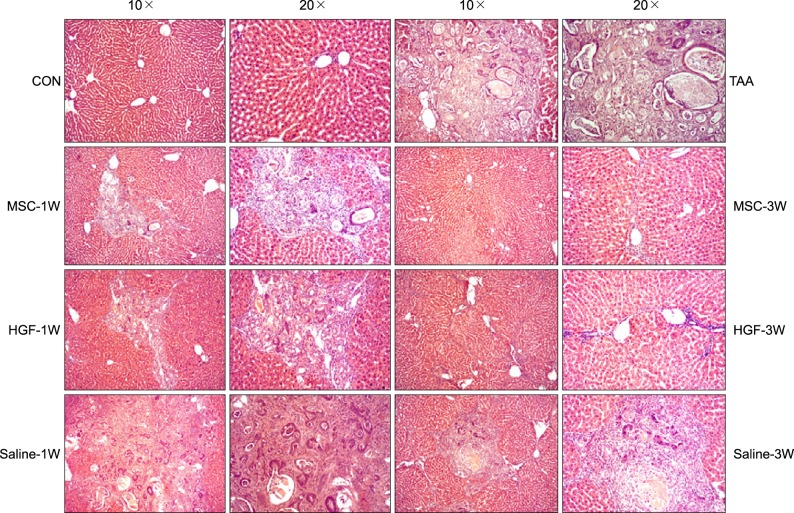
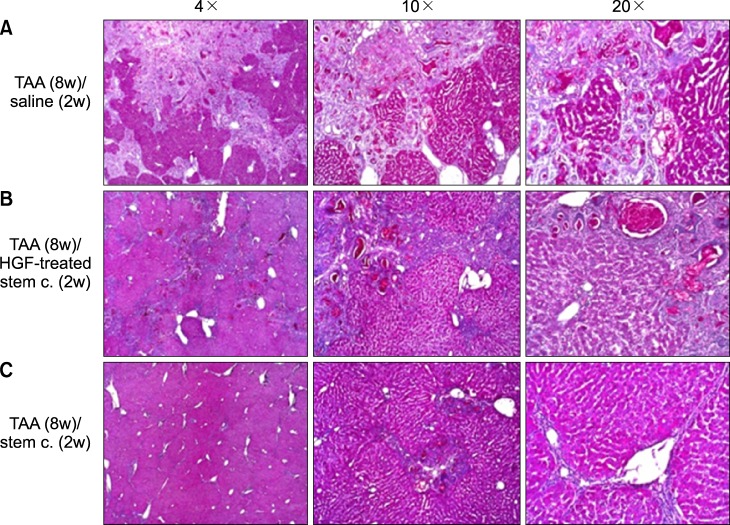
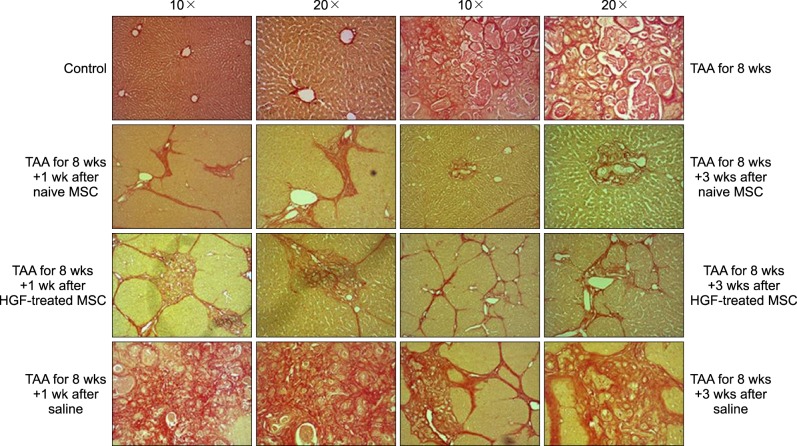
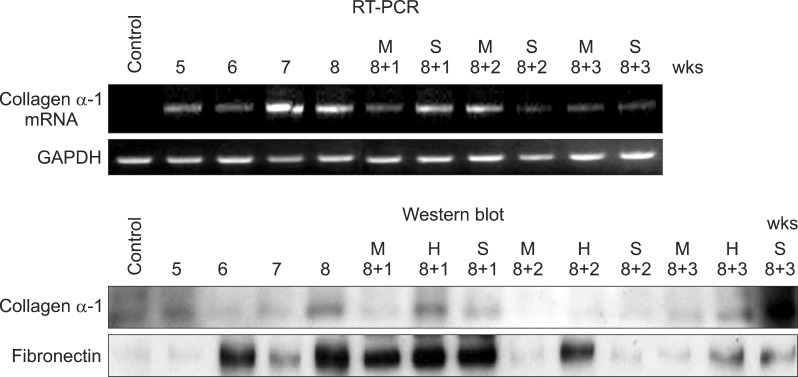
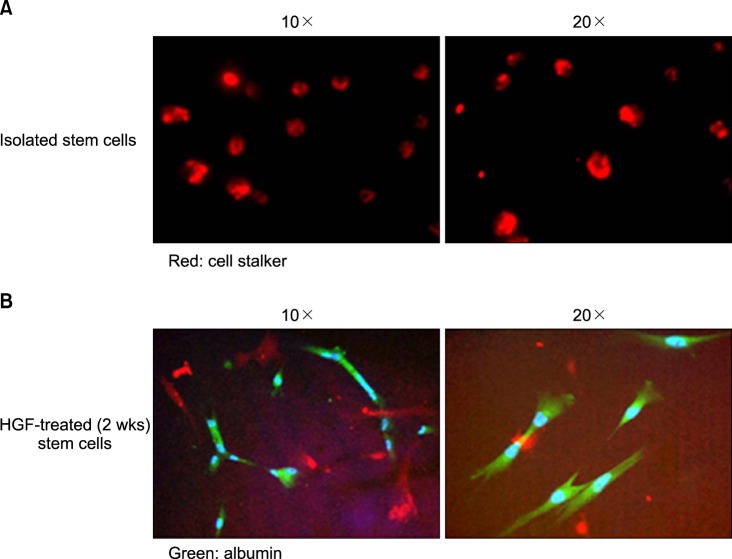
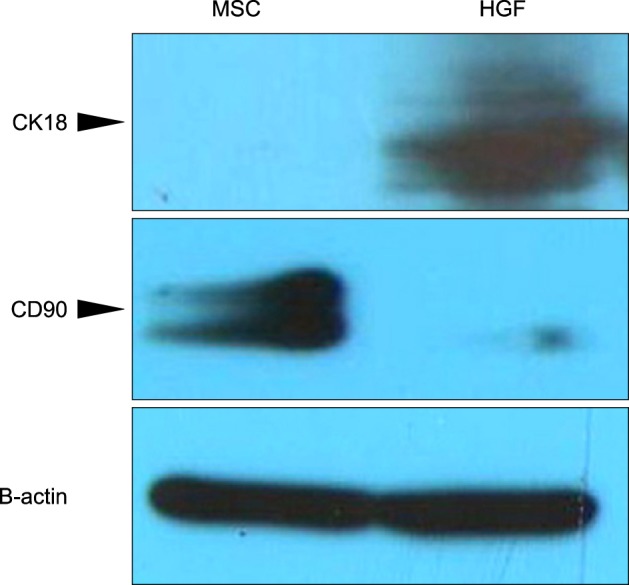
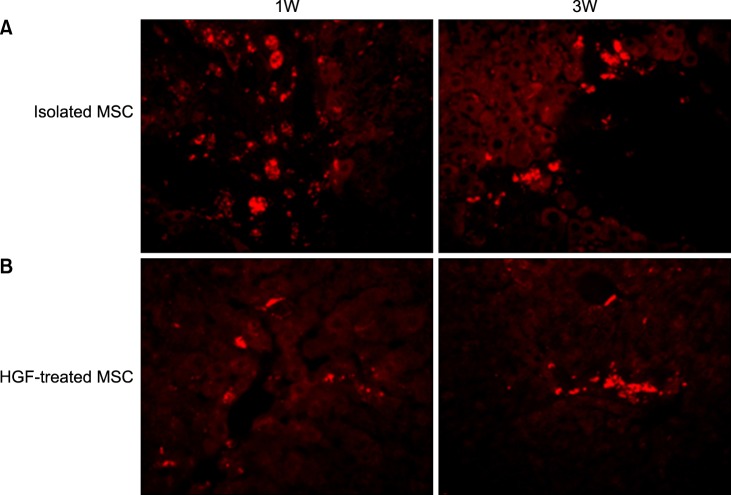
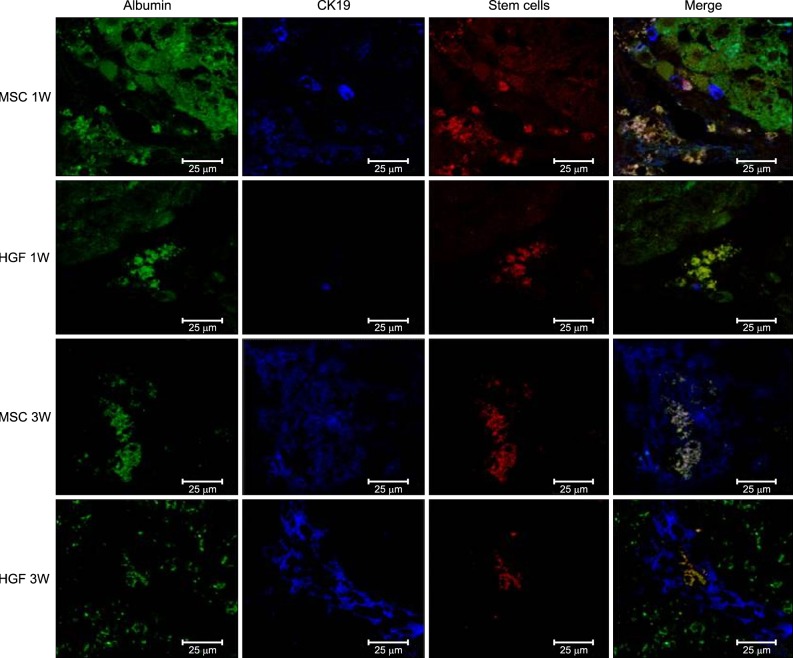
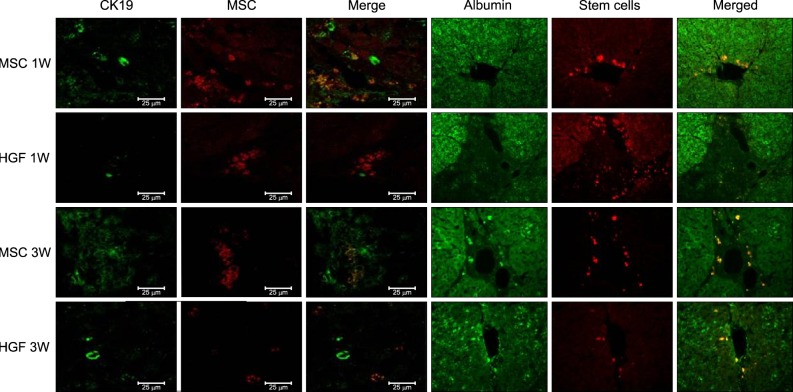
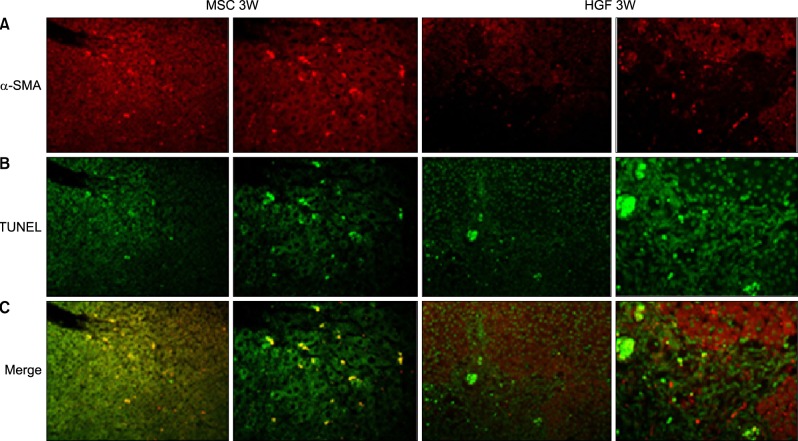
Severe hepatic fibrosis and hepatocyte destruction were detected in the control group. Less hepatic cirrhosis and collagen formation, more hepatocyte regeneration and glycogen storage were detected in isolated MSCs compared to HGF-treated MSCs group, Distribution of red autofluorescence is mainly localized near the sinusoids in isolated MSCs, scattered away the sinusoids in HGF-treated MSCs group. MSCs transdifferentiated into CK-19 postive Oval cells and then to albulmin-producing hepatocytes, HGF treated MSCs differentiated into hepatocyte without the intermediate oval cells phase. HGF treated MSCs became the CK18-positive, MSCs became CD 90-positive.
CONCLUSIONS
Significant hepatocyte differentiation occurred in not HGF-treated MSCs but isolated MSCs group unexpectedly. These results suggest that the beneficial effect of MSCs on in rat's model with TAA-induced cirrhosis may occur during early differentiation course of MSCs. Mature hepatocyte itself has a little effect on the accelerated differentiation and functional capacity of hepatic lineage cell-line.
Keyword
MeSH Terms
Figure
Reference
-
1. Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997; 92:103–112. PMID: 9059310.
Article2. Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003; 327:143–147. PMID: 12869458.
Article3. Carvalho AB, Quintanilha LF, Dias JV, et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008; 26:1307–1314. PMID: 18308943.
Article4. Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000; 290:1779–1782. PMID: 11099419.
Article5. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. PMID: 10102814.
Article6. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000; 113:1161–1166. PMID: 10704367.
Article7. Aurich I, Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007; 56:405–415. PMID: 16928726.
Article8. Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005; 106:756–763. PMID: 15817682.
Article9. Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999; 284:1168–1170. PMID: 10325227.
Article10. Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005; 11:3431–3440. PMID: 15948250.
Article11. Zheng JF, Liang LJ. Intra-portal transplantation of bone marrow stromal cells ameliorates liver fibrosis in mice. Hepatobiliary Pancreat Dis Int. 2008; 7:264–270. PMID: 18522880.
Article12. Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005; 328:258–264. PMID: 15670778.
Article13. Bian L, Guo ZK, Wang HX, et al. In vitro and in vivo immunosuppressive characteristics of hepatocyte growth factor-modified murine mesenchymal stem cells. In Vivo. 2009; 23:21–27. PMID: 19368120.14. Constantinou MA, Theocharis SE, Mikros E. Application of metabonomics on an experimental model of fibrosis and cirrhosis induced by thioacetamide in rats. Toxicol Appl Pharmacol. 2007; 218:11–19. PMID: 17113614.
Article15. Yu Y, Lu L, Qian X, et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010; 19:903–914. PMID: 20025519.
Article16. Roos F, Terrell TG, Godowski PJ, et al. Reduction of alpha-naphthylisothiocyanate-induced hepatotoxicity by recombinant human hepatocyte growth factor. Endocrinology. 1992; 131:2540–2544. PMID: 1446596.
Article17. Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006; 29:431–435. PMID: 16763914.
Article18. Strom SC, Bruzzone P, Cai H, et al. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006; 15(Suppl 1):S105–S110. PMID: 16826802.
Article19. Oyagi S, Hirose M, Kojima M, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006; 44:742–748. PMID: 16469408.
Article20. Harris RG, Herzog EL, Bruscia EM, et al. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004; 305:90–93. PMID: 15232107.
Article21. Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105:369–377. PMID: 11348593.
Article22. Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995; 129:1177–1180. PMID: 7775566.
Article23. Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996; 119:591–600. PMID: 8743556.
Article24. Rosen EM, Goldberg ID. Scatter factor and angiogenesis. Adv Cancer Res. 1995; 67:257–279. PMID: 8571817.
Article25. Furlong RA, Takehara T, Taylor WG, et al. Comparison of biological and immunochemical properties indicates that scatter factor and hepatocyte growth factor are indistinguishable. J Cell Sci. 1991; 100:173–177. PMID: 1839027.
Article26. Nusrat A, Parkos CA, Bacarra AE, et al. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest. 1994; 93:2056–2065. PMID: 8182137.
Article27. Montesano R, Matsumoto K, Nakamura T, et al. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991; 67:901–908. PMID: 1835669.
Article28. Rosen EM, Nigam SK, Goldberg ID. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994; 127:1783–1787. PMID: 7806559.
Article29. Shiota G, Wang TC, Nakamura T, et al. Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. Hepatology. 1994; 19:962–972. PMID: 8138271.
Article30. Dong XJ, Zhang H, Pan RL, et al. Identification of cytokines involved in hepatic differentiation of mBM-MSCs under liver-injury conditions. World J Gastroenterol. 2010; 16:3267–3278. PMID: 20614482.
Article31. Nishino M, Iimuro Y, Ueki T, et al. Hepatocyte growth factor improves survival after partial hepatectomy in cirrhotic rats suppressing apoptosis of hepatocytes. Surgery. 2008; 144:374–384. PMID: 18707036.
Article32. Yu Y, Lu L, Qian X, et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010; 19:903–914. PMID: 20025519.
Article33. Kaido T, Seto S, Yamaoka S, et al. Perioperative continuous hepatocyte growth factor supply prevents postoperative liver failure in rats with liver cirrhosis. J Surg Res. 1998; 74:173–178. PMID: 9587357.
Article34. Yagi H, Parekkadan B, Suganuma K, et al. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 2009; 15:3377–3388. PMID: 19397469.
Article35. Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007; 22(Suppl 1):S73–S78. PMID: 17567473.
Article36. Ren H, Zhao Q, Cheng T, et al. No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy. 2010; 12:371–383. PMID: 20184502.
Article37. Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009; 4:e6657. PMID: 19684854.
Article38. Qihao Z, Xigu C, Guanghui C, et al. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007; 26:497–503. PMID: 17630854.
Article39. Lange C, Bruns H, Kluth D, et al. Hepatocytic differentiation of mesenchymal stem cells in cocultures with fetal liver cells. World J Gastroenterol. 2006; 12:2394–2397. PMID: 16688831.
Article40. Deng X, Chen YX, Zhang X, et al. Hepatic stellate cells modulate the differentiation of bone marrow mesenchymal stem cells into hepatocyte-like cells. J Cell Physiol. 2008; 217:138–144. PMID: 18484094.
Article41. Fang B, Shi M, Liao L, et al. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004; 78:83–88. PMID: 15257043.
Article42. Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003; 100:8407–8411. PMID: 12815096.
Article43. Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009; 15:484–495. PMID: 19399744.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Liver Stem Cell Transplantation Alleviates Liver Fibrosis in a Rat Model of CCl 4 -Induced Liver Fibrosis
- Effect of Pentoxifylline on Liver Fibrosis and Cell Cycle Related Proteins in Thioacetamide-Induced Rat Cirrhosis
- Stem cell exosomes: new hope and future potential for relieving liver fibrosis
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy