Korean J Lab Med.
2006 Apr;26(2):77-80. 10.3343/kjlm.2006.26.2.77.
Three Cases of Pseudoeosinophilia Associated with Malaria Determined in the Sysmex XE-2100 Automated Hematology Analyzer
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Chung-Ang University, Seoul, Korea. chayoung@cau.ac.kr
- KMID: 2238894
- DOI: http://doi.org/10.3343/kjlm.2006.26.2.77
Abstract
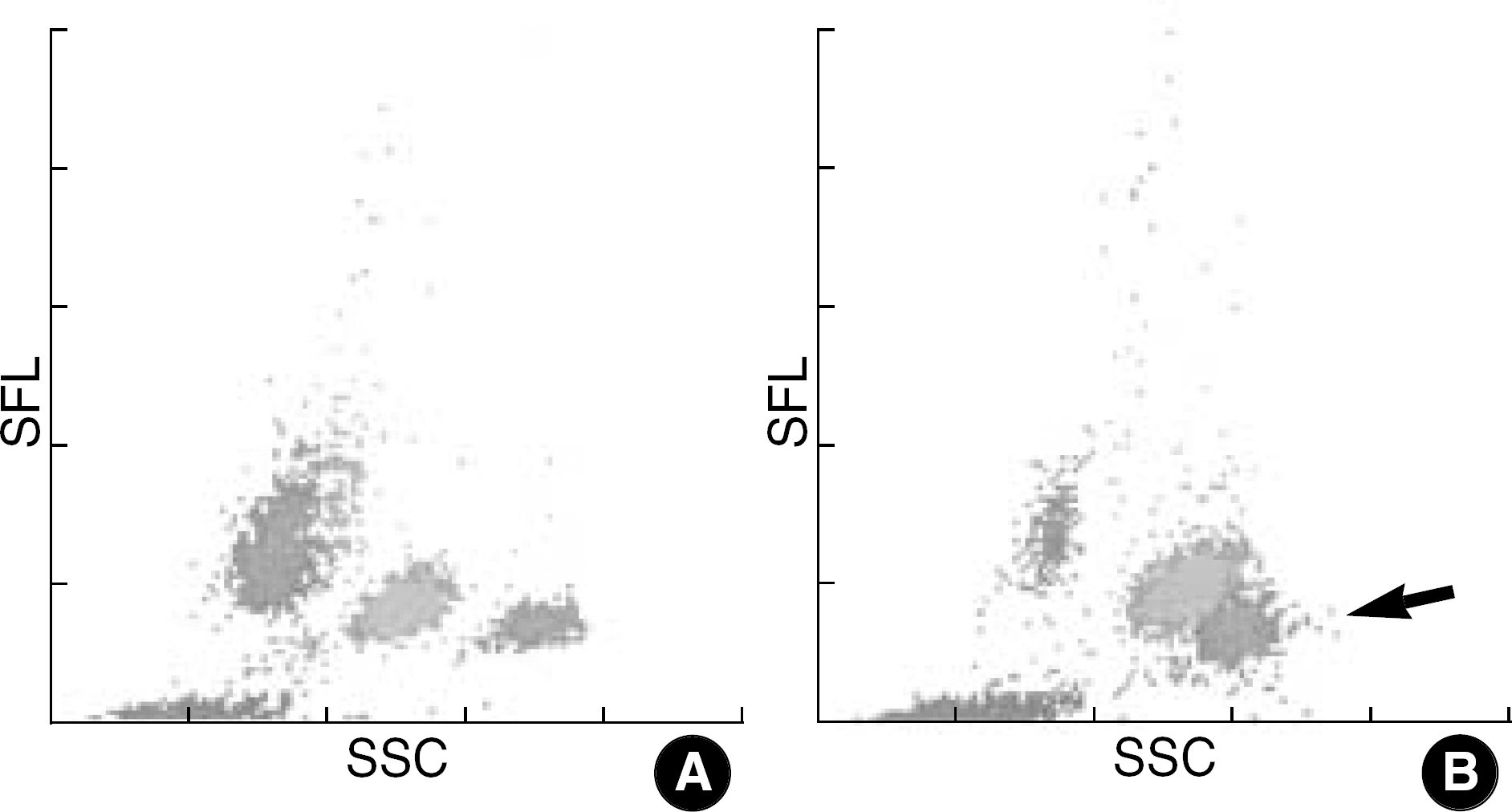
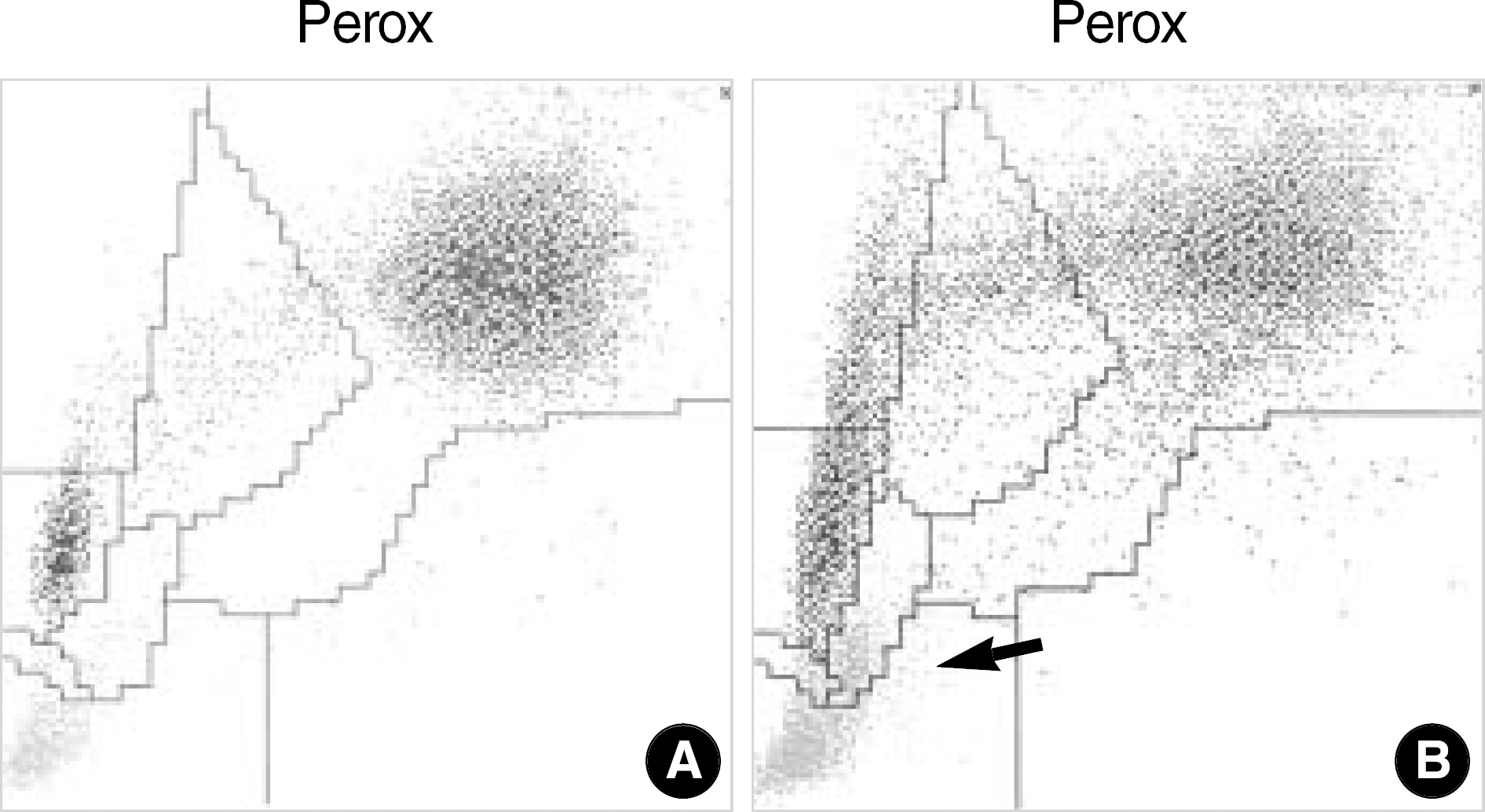
- In Korea, the incidence of malaria has been increasing in the civilian population and in the areas previously considered as noninfected. Malaria can be suspected based on the patient's symptoms and the physical findings at examination. However, for a definitive diagnosis to be made, the malaria parasites or their components must be demonstrated by laboratory tests, which will take time and require expertise. Since general screening tests, such as a complete blood cell count, are always done for patients with a fever, it can be expected that the attention of laboratory hematologists drawn to any abnormalities found in automated hematology analyzers can help reduce delays in the diagnosis of malaria even if such a diagnosis was not initially considered. We report three cases of malaria that had thrombocytopenia and pseudoeosinophilia shown in the Sysmex XE-2100 (TOA Medical Electronics, Kobe, Japan) automated hematology analyzer. It is feasible that the pseudoeosinophilia presented as a result of hemozoin-containing white blood cells may contribute to the diagnosis of malaria, especially for patients unsuspected of the disease.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kang SB, So JW, Oh KY, Suh DY. A case of cerebral malaria. Korean J Medicine. 1984; 27:1117–382.2. Park KI, Park HD, Han DG, Kim KY, Min DY, Soh CT. A case of congenital malaria. Korean J Parasitol. 1984; 22:72–7.
Article3. Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasitol. 1994; 32:195–200.
Article4. Cho SY, Kong Y, Park SM, Lee JS, Lim YA, Chae SL, et al. Two vivax malaria cases detected in Korea. Korean J Parasitol. 1994; 32:281–4.
Article5. Chai JY. Reemerging Plasmodium vivax Malaria in the Republic of Korea. Medical Postgraduate. 2003; 31:321–7.6. Koo KB, Cho NH, Kim SH, Won YJ, Cho HS. The clinical analysis of 79 cases of indigenous malaria in Myongji hospital during 4 years. J Korean Acad Fam Med. 2004; 25:403–10.7. Song HH, O SO, Kim SH, Moon SH, Kim JB, Yoon JW, et al. Clinical features of Plasmodium vivax malaria. Korean J Intern Med. 2003; 18:220–4.8. Oh J, Kim M, Lim J, Oh E, Lee J, Lee H, et al. Immunological alterations of tertian malaria in Korea. Korean J Clin Pathol. 2000; 20:178–83.9. Rodriguez-Morales AJ, Sanchez E, Vargas M, Piccolo C, Colina R, Arria M. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr. 2006; 52:49–51.10. Jadhav UM, Patkar VS, Kadam NN. Thrombocytopenia in malaria-correlation with type and severity of malaria. J Assoc Physicians India. 2004; 52:615–8.11. Scott CS, Van Zyl D, Ho E, Ruivo L, Mendelow B, Coetzer TL. Thrombocytopenia in patients with malaria: automated analysis of optical platelet counts and platelet clumps with the Cell Dyn CD4000 analyser. Clin Lab Haematol. 2002; 24:295–302.
Article12. Erel O, Vural H, Aksoy N, Aslan G, Ulukanligil M. Oxidative stress of platelets and thrombocytopenia in patients with vivax malaria. Clin Biochem. 2001; 34:341–4.
Article13. Chai JY. Reemerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999; 37:129–43.14. Shanks GD, Wilairatanaporn C. Eosinophilic response to falciparum malaria infections. Southeast Asian J Trop Med Public Health. 1992; 23:795–7.15. Camacho LH, Wilairatana P, Weiss G, Mercader MA, Brittenham GM, Looareesuwan S, et al. The eosinophilic response and haematological recovery after treatment for Plasmodium falciparum malaria. Trop Med Int Health. 1999; 4:471–5.16. Rosenthal PJ, Meshnick SR. Hemoglobin catabolism and iron utilization by malaria parasites. Mol Biochem Parasitol. 1996; 83:131–9.
Article17. Noland GS, Briones N, Sullivan DJ Jr. The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol Biochem Parasitol. 2003; 130:91–9.18. Huh J, Jung J, Yoon H, Chung W. Pseudoeosinophilia associated with malaria infection determined in the Sysmex XE-2100 hematology analyzer. Ann Hematol. 2005; 84:400–2.
Article19. Scott CS, Van Zyl D, Ho E, Ruivo L, Kunz D, Coetzer TL. Patterns of pseudo-reticulocytosis in malaria: fluorescent analysis with the Cell-Dyn CD 4000. Clin Lab Haematol. 2002; 24:15–20.20. Wever PC, Henskens YM, Kager PA, Dankert J, van Gool T. Detection of Imported Malaria with the Cell-Dyn 4000 Hematology Analyzer. J Clin Microbiol. 2002; 40:4729–31.
Article21. Hoffmann JJ, Pennings JM. Pseudo-reticulocytosis as a result of malaria parasites. Clin Lab Haematol. 1999; 21:257–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Mindray BC-6200 Hematology Analyzer; Comparison with Sysmex XE-2100 and Manual Microscopy
- Performance Evaluation of the Beckman Coulter DxH 900 Automated Hematology Analyzer
- Evaluation of the Automated Hematology Analyzer Sysmex XN-2000 and the Accuracy of Differential Leukocyte Counts Using the Low WBC Mode
- Evaluation of a portable hemoglobin photometer for assessment of intra-operative hemoglobin concentrations
- Evaluation of Platelet count by the CELL-DYN Sapphire CD61 Immunoplatelet Method in Patients with Hematologic Diseases Receiving Chemotherapy