Korean Circ J.
2008 Feb;38(2):73-79. 10.4070/kcj.2008.38.2.73.
Therapeutic Angiogenesis: The Pros and Cons and the Future
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea. jkryu@cu.ac.kr
- KMID: 2225838
- DOI: http://doi.org/10.4070/kcj.2008.38.2.73
Abstract
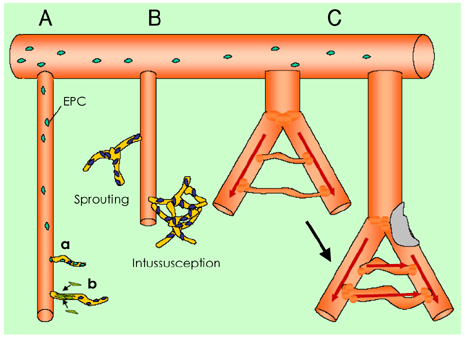
- Despite the improvements in medical, surgical and endovascular therapies, vascular disease is still a significant, critical clinical problem. The advances in understanding the mechanisms of neovascularization and the accumulated experiences of successful therapeutic application in animal models have raised expectations for therapeutic angiogenesis as a promising treatment option. However, the large, double-blinded, controlled clinical trials using therapeutic agent in the form of protein, naked DNA or viral gene therapy have failed to show clinical benefit. Nevertheless, by this time, cell based therapeutic angiogenesis has raised a promising option for the treatment of ischemic diseases. This article summarizes the essential preclinical research and major clinical trials on therapeutic angiogenesis, and it deals with several issues related to the failure of the clinical trials. Future directions in the realm of therapeutic angiogenesis are also described with focusing on cell based therapy.
Keyword
Figure
Reference
-
1. Kim HJ, Kim DK. Angiogenic gene therapy in patients with ischemic cardiovascular diseases. Korean Circ J. 2003. 33:7–14.2. Choi JH, Kim DK. Therapeutic angiogenesis for cardiovascular diseases: the present and future. Korean Circ J. 2003. 33:739–745.3. Simons M. Angiogenesis: where do we stand now? Circulation. 2005. 111:1556–1566.4. Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus. Circulation. 2000. 102:e73–e86.5. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008. 28:208–216.6. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003. 9:653–660.7. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003. 9:677–684.8. Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis). J Pathol. 2000. 190:338–342.9. Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004. 287:C572–C579.10. Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004. 94:230–238.11. Vartanian SM, Sarkar R. Therapeutic angiogenesis. Vasc Endovascular Surg. 2007. 41:173–185.12. Yanagisawa MA, Uchida Y, Nakamura F, et al. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992. 257:1401–1403.13. Takeshita S, Pu LQ, Stein LA, et al. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994. 90:II228–II234.14. Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002. 105:788–793.15. Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003. 107:1359–1365.16. Grines CL, Watkins MW, Helmer G, et al. Angiogenic gene therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002. 105:1291–1297.17. Grines CL, Watkins MW, Mahmarian JJ, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003. 42:1339–1347.18. Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study. J Am Coll Cardiol. 2005. 45:982–988.19. Rajagopalan S, Mohler ER 3rd, Lederman RJ, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003. 108:1933–1938.20. Kusumanto YH, van Weel V, Mulder NH, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006. 17:683–691.21. Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002. 105:788–793.22. Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002. 34:775–787.23. Lee CW, Stabile E, Kinnaird T, et al. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004. 43:474–482.24. Post MJ, Simons M. Gene therapy versus protein-based therapy: a matter of pharmacokinetics. Drug Discov Today. 2001. 6:769–770.25. Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002. 8:831–840.26. Lu E, Wagner WR, Schellenberger U, et al. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003. 108:97–103.27. Pearlman JD, Laham RJ, Post M, Leiner T, Simons M. Medical imaging techniques in the evaluation of strategies for therapeutic angiogenesis. Curr Pharm Des. 2002. 8:1467–1496.28. Viswamitra S, Higgins CB, Meacham DF, Mehta JL. Magnetic resonance imaging in myocardial ischemia. Curr Opin Cardiol. 2004. 19:510–516.29. Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002. 22:1567–1572.30. Sata M. Circulating vascular progenitor cells contribute to vascular repair, remodeling, and lesion formation. Trends Cardiovasc Med. 2003. 13:249–253.31. Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003. 108:2710–2715.32. Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003. 93:e17–e24.33. Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001. 7:425–429.34. Kang HJ, Kim HS, Zhang SY, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004. 363:751–756.35. Yla-Hurttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003. 9:694–701.36. Corti R, Badimon J, Mizsei G, et al. Real time magnetic resonance guided endomyocardial local delivery. Heart. 2005. 91:348–353.37. Yoon CH, Seo JB, Hur J, et al. Characterization of two types of endothelial progenitor cells (EPC). Korean Circ J. 2004. 34:304–313.38. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004. 95:343–353.39. Cheon IS, Choi JH, Kim KL, et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Korean Circ J. 2004. 34:1033–1042.40. Joe YA, Baek SH, Park HE, et al. In vitro differentiation of endothelial precursor cells derived form umbilical cord blood. Korean Circ J. 2002. 32:646–654.41. Assmus B, Schachinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation. 2002. 106:3009–3017.42. Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004. 94:92–95.43. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.44. Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006. 367:113–121.45. Lunde K, Solheim S, Aakhus S, et al. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial: intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J. 2005. 39:150–158.46. Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. 355:1210–1221.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Robotic Thyroidectomy: Pros and Cons of Various Surgical Approaches
- Pros & Cons
- A study of the Stage of Change and Decisional balance: Exercise Acquisition, Smoking Cessation, Mammography Screening and Kegel's Exercise Acquisition in Korea
- New Medical Education System-Pros and Cons
- Reimmunization of varicella vaccination: Pros and cons