Korean Circ J.
2013 Nov;43(11):752-760. 10.4070/kcj.2013.43.11.752.
Incidence, Diagnosis and Prognosis of Cardiac Amyloidosis
- Affiliations
-
- 1Cardiovascular Center, Seoul National University Hospital, Seoul, Korea. dwsohn@snu.ac.kr
- KMID: 2224807
- DOI: http://doi.org/10.4070/kcj.2013.43.11.752
Abstract
- BACKGROUND AND OBJECTIVES
Cardiac involvement is frequent in systemic amyloidosis and is the most important determinant of the clinical outcome. The aims of this study were to assess the incidence and prognosis of cardiac amyloidosis and discuss the diagnostic issues related to cardiac amyloidosis.
SUBJECTS AND METHODS
We retrospectively studied all patients diagnosed with systemic amyloidosis who presented to our institution from January 1999 to December 2011.
RESULTS
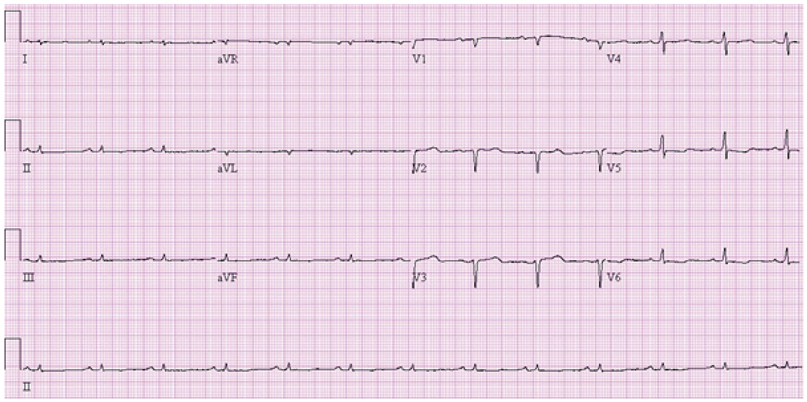
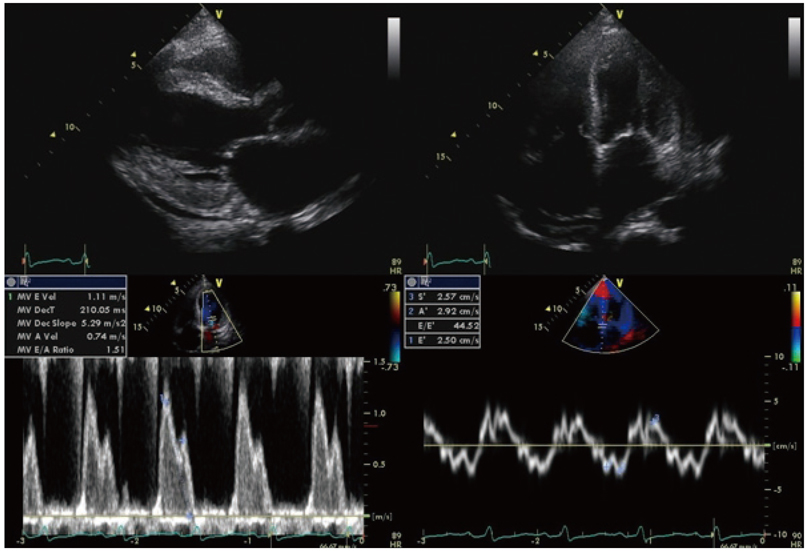
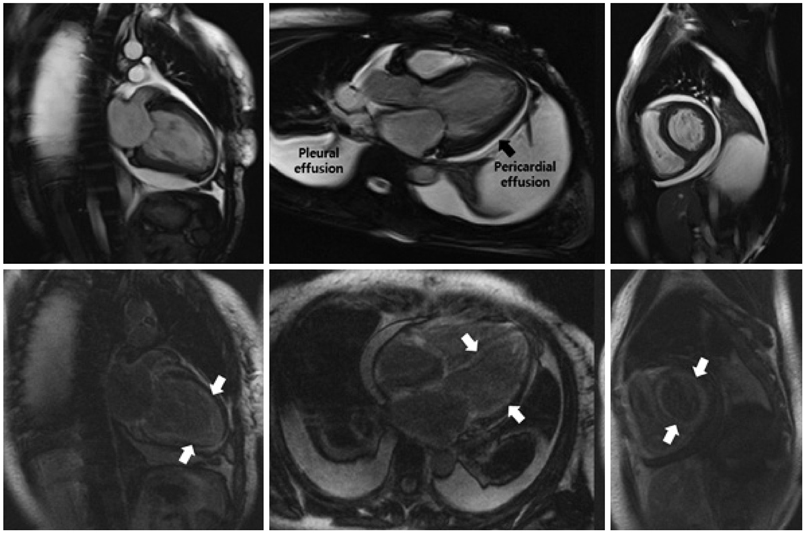
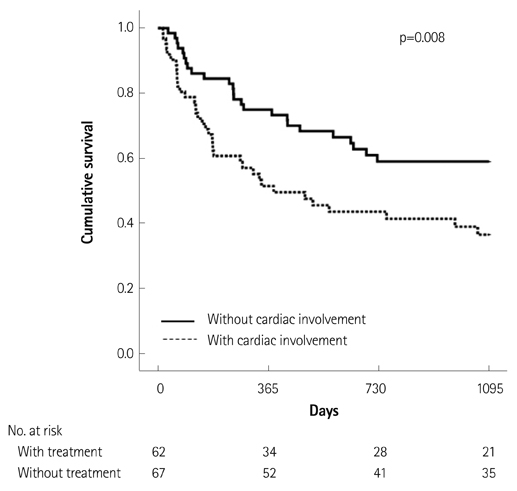
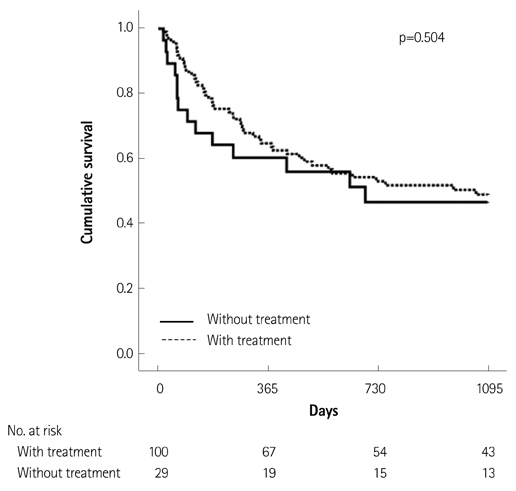
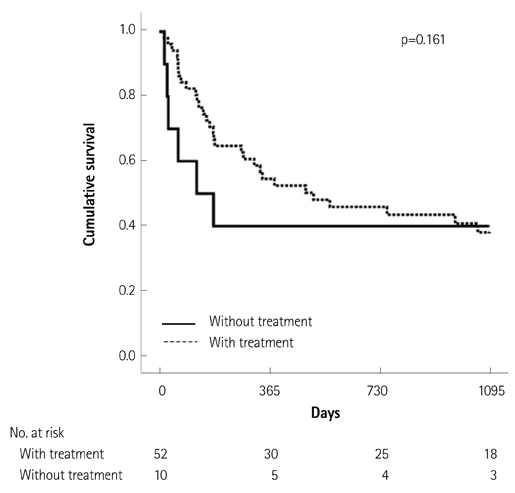
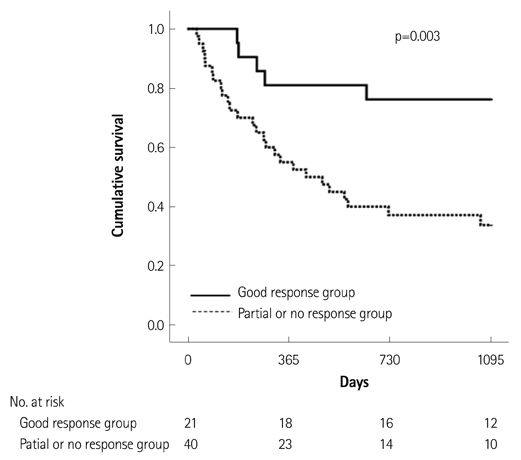
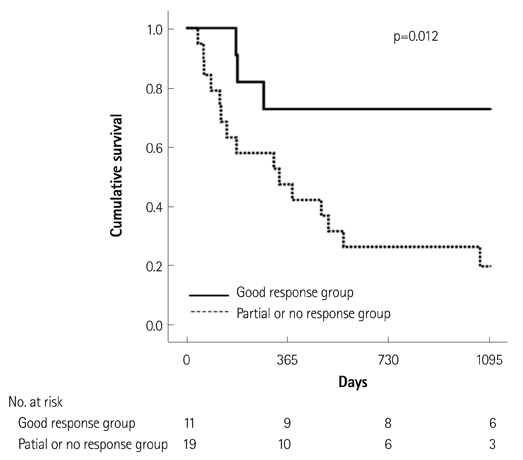
Of the 129 patients with systemic amyloidosis, cardiac amyloidosis was diagnosed in 62 patients. At the 3 years' follow-up of the patients with systemic amyloidosis, there was a statistically significant difference in mortality between patients with cardiac amyloidosis and the rest of the patients (58.1% vs. 37.3%, p=0.008). In the Cox proportional hazard model, old age {hazard ratio (HR) 18.336, p=0.006}, elevation of cardiac troponin I (cTNI) (HR 13.246, p=0.020), left ventricular (LV) systolic dysfunction (HR 5.137, p=0.041) and diastolic dysfunction (HR 64.595, p=0.022) were independently associated with survival in cardiac amyloidosis. In the diagnosis of monoclonal gammopathy, serum or urine protein electrophoresis was not sensitive enough to be used clinically compared to serum free light chain assay (35.8% vs. 96.4%).
CONCLUSION
In systemic amyloidosis, cardiac involvement was the most important determinant of the prognosis, and old age, elevation of cTNI, LV systolic dysfunction and diastolic dysfunction were independently associated with survival in cardiac amyloidosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Merlini G, Westermark P. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med. 2004; 255:159–178.2. Westermark P, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature -- 2002. Amyloid. 2002; 9:197–200.3. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med. 1987; 82:412–414.4. Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989; 13:1017–1026.5. Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008; 51:1022–1030.6. Perugini E, Rapezzi C, Piva T, et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006; 92:343–349.7. Arbustini E, Verga L, Concardi M, Palladini G, Obici L, Merlini G. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid. 2002; 9:108–114.8. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005; 79:319–328.9. Kyle RA, Greipp PR, O'Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986; 68:220–224.10. Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001; 19:3350–3356.11. Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000; 45:107–137.12. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998; 91:141–157.13. Morris KL, Tate JR, Gill D, et al. Diagnostic and prognostic utility of the serum free light chain assay in patients with AL amyloidosis. Intern Med J. 2007; 37:456–463.14. Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005; 95:535–537.15. Cueto-Garcia L, Tajik AJ, Kyle RA, et al. Serial echocardiographic observations in patients with primary systemic amyloidosis: an introduction to the concept of early (asymptomatic) amyloid infiltration of the heart. Mayo Clin Proc. 1984; 59:589–597.16. Klein AL, Hatle LK, Taliercio CP, et al. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990; 16:1135–1141.17. Hamer JP, Janssen S, van Rijswijk MH, Lie KI. Amyloid cardiomyopathy in systemic non-hereditary amyloidosis. Clinical, echocardiographic and electrocardiographic findings in 30 patients with AA and 24 patients with AL amyloidosis. Eur Heart J. 1992; 13:623–627.18. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004; 43:410–415.19. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982; 49:9–13.20. Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010; 3:155–164.21. Kristen AV, Perz JB, Schonland SO, et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007; 9:617–624.22. Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983; 58:665–683.23. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004; 140:85–93.24. Seldin DC, Anderson JJ, Sanchorawala V, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004; 104:1888–1893.25. Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012; 30:4541–4549.26. Klein AL, Hatle LK, Taliercio CP, et al. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991; 83:808–816.27. Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003; 122:78–84.28. Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006; 107:3854–3858.29. Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003; 361:1787–1789.30. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997; 30:8–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac Amyloidosis Determines the Prognosis of Systemic Amyloidosis; Roles and Responsibilities of Cardiologist
- Contemporary Imaging Diagnosis of Cardiac Amyloidosis
- Primary Amyloidosis of the Bladder
- Isolated Tricuspid Regurgitation: Initial Manifestation of Cardiac Amyloidosis
- Hepatic amyloidosis: two cases report