J Korean Ophthalmol Soc.
2014 Mar;55(3):368-373. 10.3341/jkos.2014.55.3.368.
The Clinical Features and Progression of the Disease in Posterior Polymorphous Corneal Dystrophy (PPCD)
- Affiliations
-
- 1Department of Ophthalmology and Visual Science, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. mskim@catholic.ac.kr
- KMID: 2218264
- DOI: http://doi.org/10.3341/jkos.2014.55.3.368
Abstract
- PURPOSE
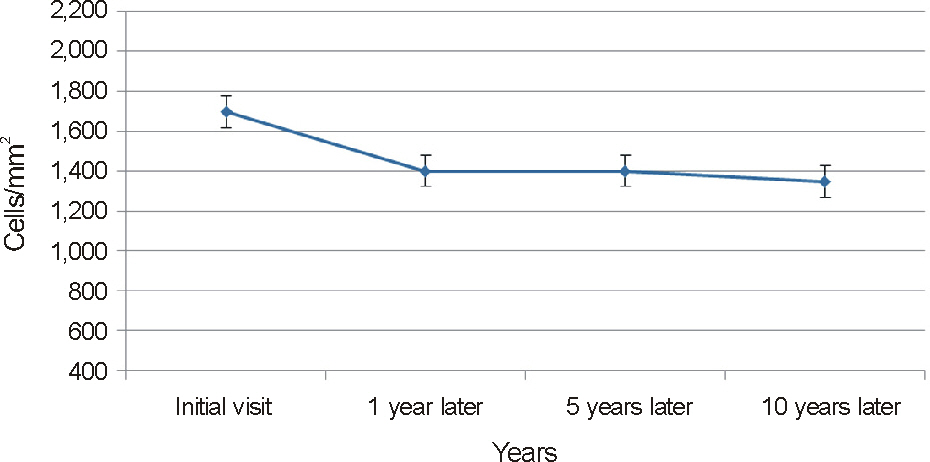
To analyze the clinical features and the relationship between endothelial cell changes and progression of posterior polymorphous corneal dystrophy (PPCD) disease by evaluating a case series of 74 eyes in 37 patients.
METHODS
From 1995 to 2012, patients were selected from those referred with a probable clinical diagnosis of PPCD to a special study group. Selection was based on the slit-lamp appearance of each case. A total of 37 patients who were diagnosed as PPCD were assessed with respect to gender, age of onset, genetic influences, and affected eyes (unilateral or bilateral). Additionally, we observed the relationship between the changes of the patients' lesions, progression of disease and the rate of loss of corneal endothelial cells.
RESULTS
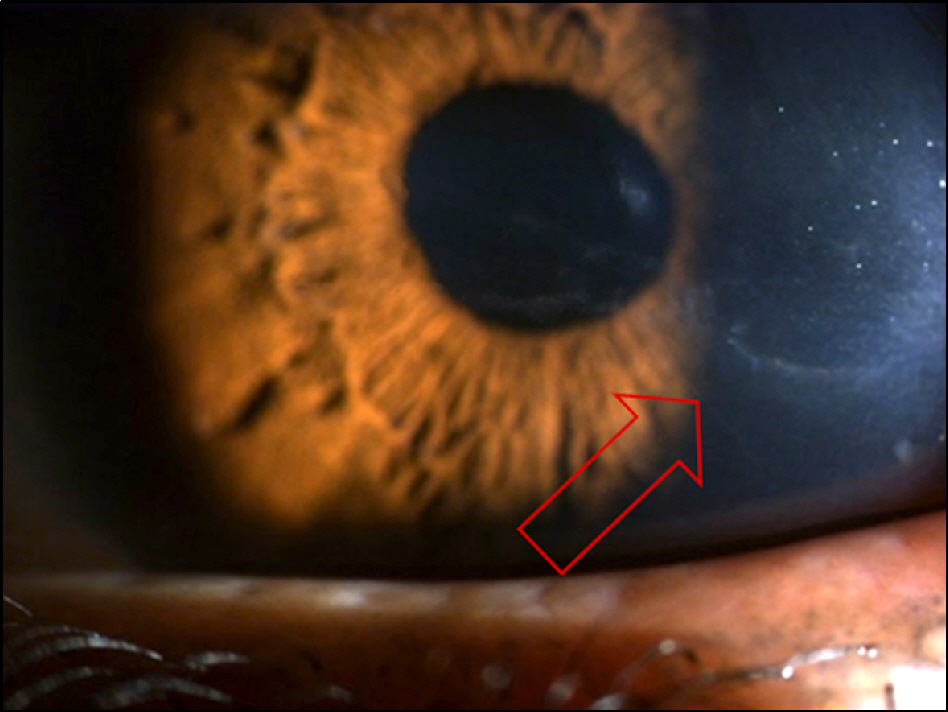
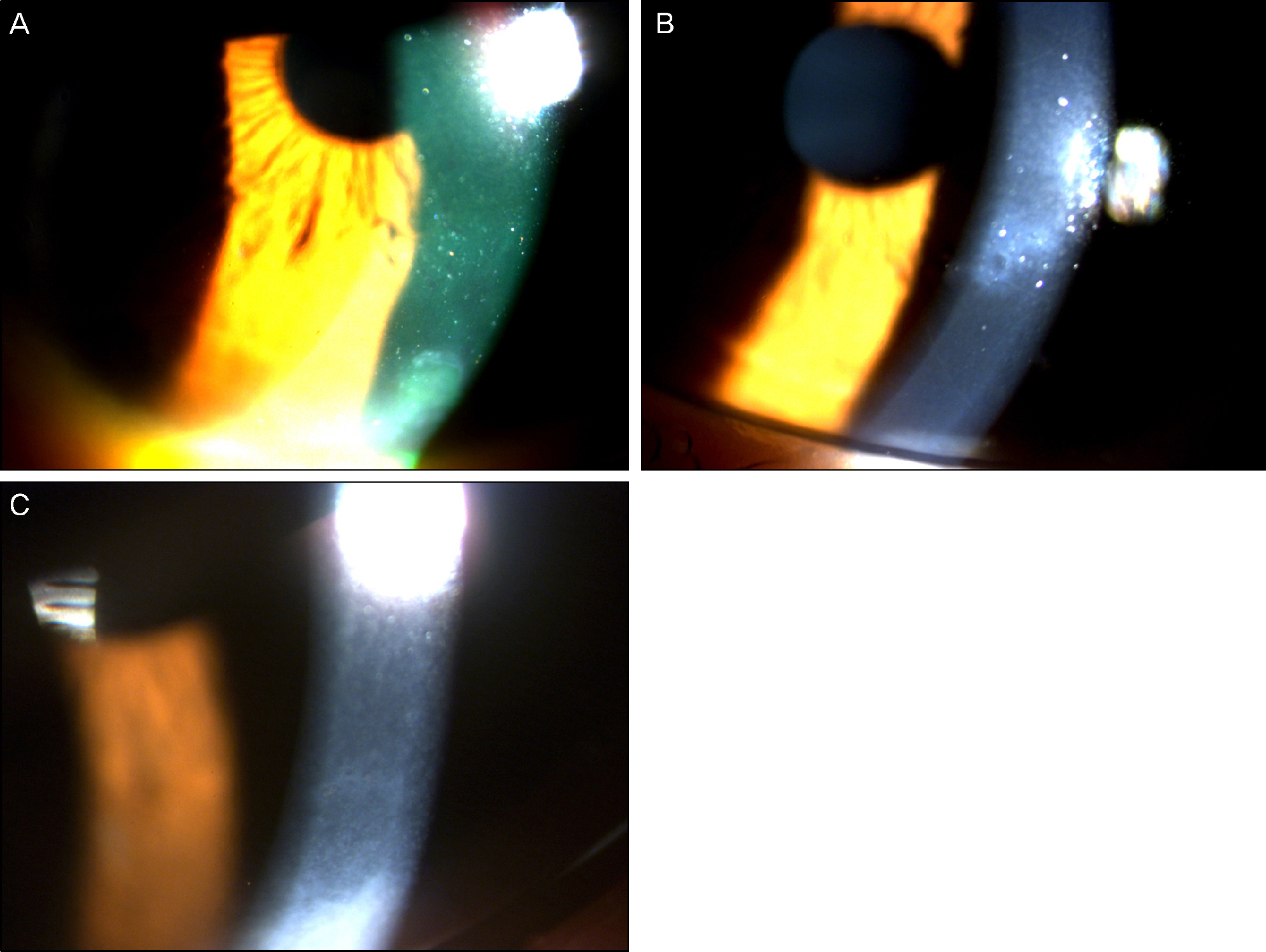
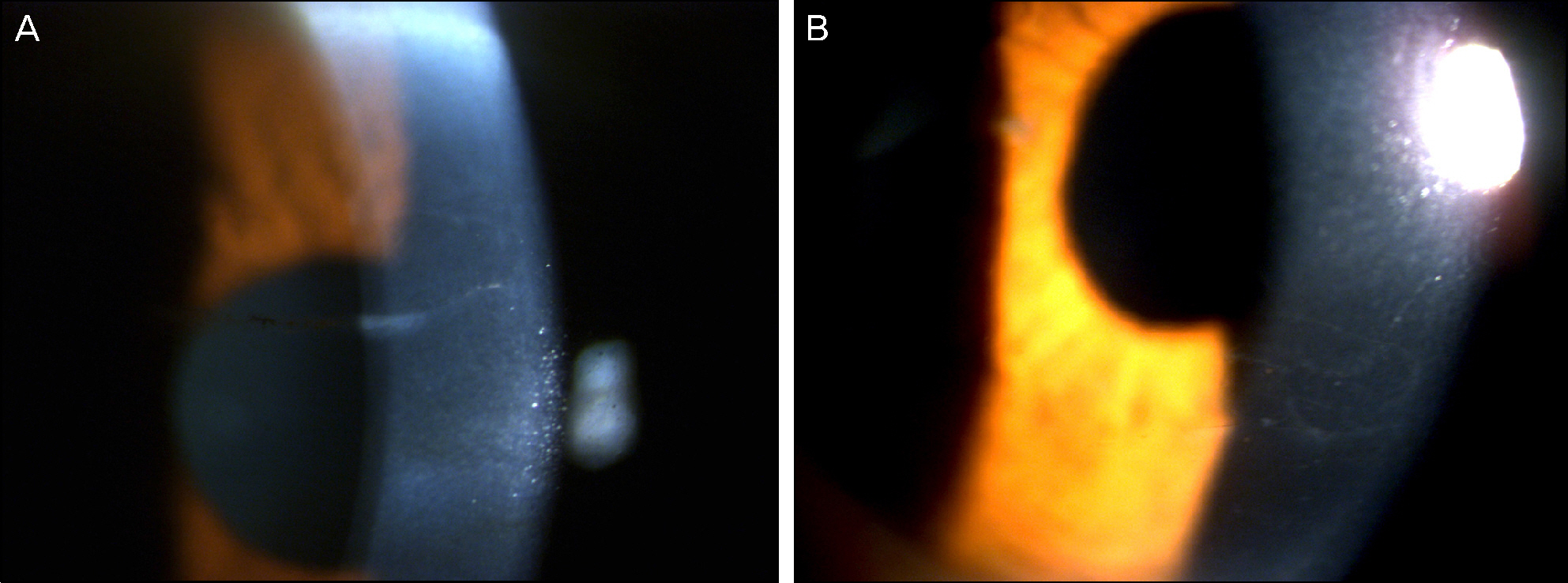
On slit lamp examination, a tram-track line appearance of posterior corneal surface was detected in a majority of patients. Most lesions lied horizontally except for 1 patient (vertically located). At the time of initial diagnosis, the patients' endothelial cell count was under 2,000 cells/mm2, a slow unilateral progressive loss of endothelial cells was observed over 10 years and there was no associated gender differences. Most patients were diagnosed after their 30's during incidental visits. No specific gene mutations were found in screening of the coding sequence of genes for mutations. Most patients were asymptomatic, although 1 patient underwent penetrating keratoplasty (PKP) due to bullous keratopathy and 2 patients had glaucoma surgery because of iridocorneal adhesion.
CONCLUSIONS
In PPCD, there was no gender difference and most lesions were unilateral. Additionally, no remarkable gene mutations were observed. When significantly different endothelial cell counts between patient's eyes were detected, a tram-track line appearance on a patient's cornea surface was observed. Some patients had corneal dysfunction and glaucoma, but the frequency was lower.
MeSH Terms
Figure
Cited by 1 articles
-
Iridocorneal Endothelial Syndrome with Features of Posterior Polymorphous Corneal Dystrophy
Jeong Ho Na, Hyo Kyung Lee
J Korean Ophthalmol Soc. 2019;60(9):909-914. doi: 10.3341/jkos.2019.60.9.909.
Reference
-
References
1. Kaufman HE, Barron BA, McDonald MB, Waltman SR. The cornea. 1st ed.New York: Churchill Livingstone;1988. p. 432–8.2. Cibis GW, Krachmer JA, Phelps CD, Weingeist TA. The clinical spectrum of posterior polymorphous dystrophy. Arch Ophthalmol. 1977; 95:1529–37.
Article3. Grayson M. The nature of hereditary deep polymorphous dystrophy of the cornea: its association with iris and anterior chamber dygenesis. Trans Am Ophthalmol Soc. 1974; 72:516–59.4. Bourgeois J, Shields MB, Thresher R. Open-angle glaucoma associated with posterior polymorphous dystrophy. A clinicopathologic study. Ophthalmology. 1984; 91:420–3.5. Babu K, Murthy KR. In vivo confocal microscopy in different types of posterior polymorphous dystrophy. Indian J Ophthalmol. 2007; 55:376–8.6. Cibis GW, Tripathi RC. The differential diagnosis of Descemet's tears (Haab's striae) and posterior polymorpous dystrophy bands. A clinicopathologic study. Ophthalmology. 1982; 89:614–20.7. Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985; 83:413–75.8. Henriquez AS, Kenyon KR, Dohlman CH, et al. Morphologic characteristics of posterior polymorphous dystrophy. A study of nine corneas and review of the literature. Surv Ophthalmol. 1984; 29:139–47.
Article9. Hanna C, Fraunfelder FT, McNair JR. An ultrastructure study of posterior polymorphous dystrophy of the cornea. Ann Ophthalmol. 1977; 9:1371–8.10. de Felice GP, Braidotti P, Viale G, et al. Posterior polymorphous dystrophy of the cornea. An ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1985; 223:265–71.11. Polack FM, Bourne WM, Forstot SL, Yamaguchi T. Scanning electron microscopy of posterior polymorphous corneal dystrophy. Am J Ophthalmol. 1980; 89:575–84.
Article12. Boruchoff SA, Kuwabara T. Electron microscopy of posterior polymorphous degeneration. Am J Ophthalmol. 1971; 72:879–87.
Article13. Rodrigues MM, Sun TT, Krachmer J, Newsome D. Epithelialization of the corneal endothelium in posterior polymorphous dystrophy. Invest Ophthalmol Vis Sci. 1980; 19:832–5.14. Rodrigues MM, Waring GO, Laibson PR, Weinreb S. Endothelial alterations in congenital corneal dystrophies. Am J Ophthalmol. 1975; 80:678–89.
Article15. Threlkeld AB, Green WR, Quigley HA, et al. A clinicopathologic study of posterior polymorphous dystrophy:implications for patho-genetic mechanism of the associated glaucoma. Trans Am Ophthalmol Soc. 1994; 92:133–65.16. Goldman RD, Zackroff RV, Steinert PM. Intermediate filaments: an overview. Goldman RD, Steinert PM, editors. Cellular and Molecular Biology of Intermediate Filaments. New York, NY: Plenum Press;1990. p. 3–17.17. Patel DV, Grupcheva CN, McGhee CN. In vivo confocal microscopy of posterior polymorphous dystrophy. Cornea. 2005; 24:550–4.
Article18. Cibis GW, Krachmer JH, Phelps CD, et al. Iridocorneal adhesions in posterior polymorphous corneal dystrophy. Trans Am Ophthalmol Soc. 1976; 81:770–7.19. Aldave AJ, Yellore VS, Principe AH, et al. Candidate gene screening for posterior polymorphous dystrophy. Cornea. 2005; 24:151–5.
Article20. Héon E, Greenberg A, Kopp KK, et al. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002; 11:1029–36.21. Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001; 10:2415–23.
Article22. McCartney AC, Kirkness CM. Comparison between posterior polymorphous dystrophy and congenital hereditary endothelial dystrophy of the cornea. Eye (Lond). 1988; 2(Pt 1):63–70.
Article23. Park IC, Chung SK, Myong YW, Rhee SW. A case of posterior polymorphous dystrophy. J Korean Ophthalmol Soc. 1991; 32:1020–3.24. Koeppe L. Klinische Beobachtunigen mit der Nernstspaltlampe und dem Hornhautmikroskop. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1916; 91:375–9.25. Waring GO 3rd, Rodrigues MM, Laibson PR. Corneal dystrophies. II. Endothelial dystrophies. Surv Ophthalmol. 1978; 23:147–68.
Article26. Shields MB. Progressive essential iris atrophy, Chandler's syndrome, and the iris nevus (Cogan-Reese) syndrome: a spectrum of disease. Surv Ophthalmol. 1979; 24:3–20.
Article27. Weiss JS, Møller HU, Lisch W, et al. The IC3D classification of the corneal dystrophies. Cornea. 2008; 27(Suppl 2):S1–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posterior Polymorphous Corneal Dystrophy (PPCD) Combined with Traumatic Descemet's Membrane Fold
- A Case of Posterior Polymorphous Dystrophy
- Confocal Microscopic Findings in Posterior Polymorphous Corneal Dystrophy
- Iridocorneal Endothelial Syndrome with Features of Posterior Polymorphous Corneal Dystrophy
- Granular Corneal Dystrophy