J Korean Ophthalmol Soc.
2015 Oct;56(10):1511-1519. 10.3341/jkos.2015.56.10.1511.
Effect of Cysteamine on Human Peripheral Blood Mononuclear Cells-Chemically Injured Keratocytes Reaction
- Affiliations
-
- 1Department of Ophthalmology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. schinn@hanmail.net
- 2Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Rhee's Eye Hospital, Daejeon, Korea.
- KMID: 2214409
- DOI: http://doi.org/10.3341/jkos.2015.56.10.1511
Abstract
- PURPOSE
To investigate the effect of cysteamine on mixed peripheral blood mononuclear cells (PBMCs)-chemically injured keratocytes reaction (mixed lymphocyte-keratocyte reaction; MLKR).
METHODS
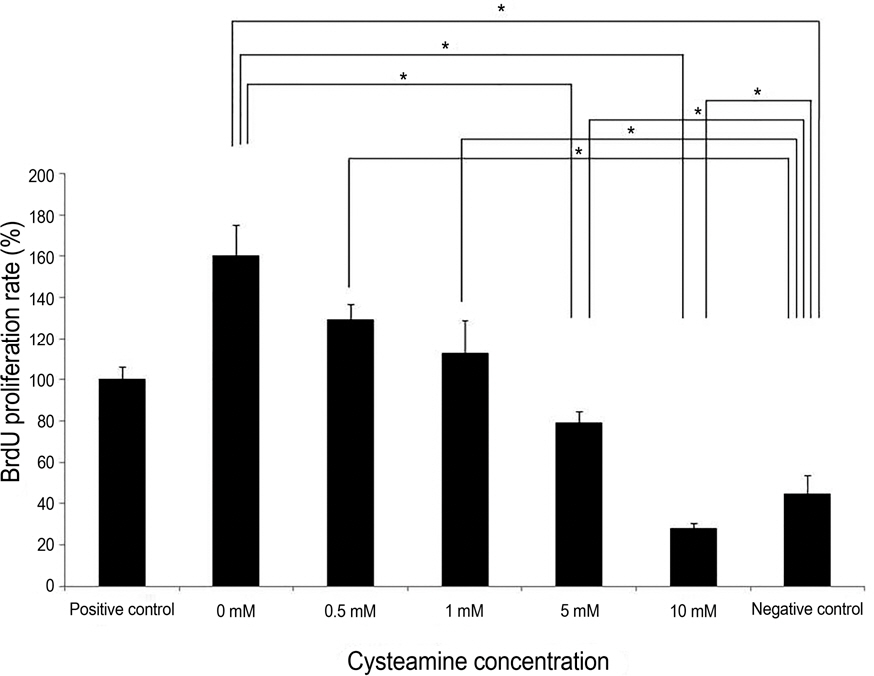
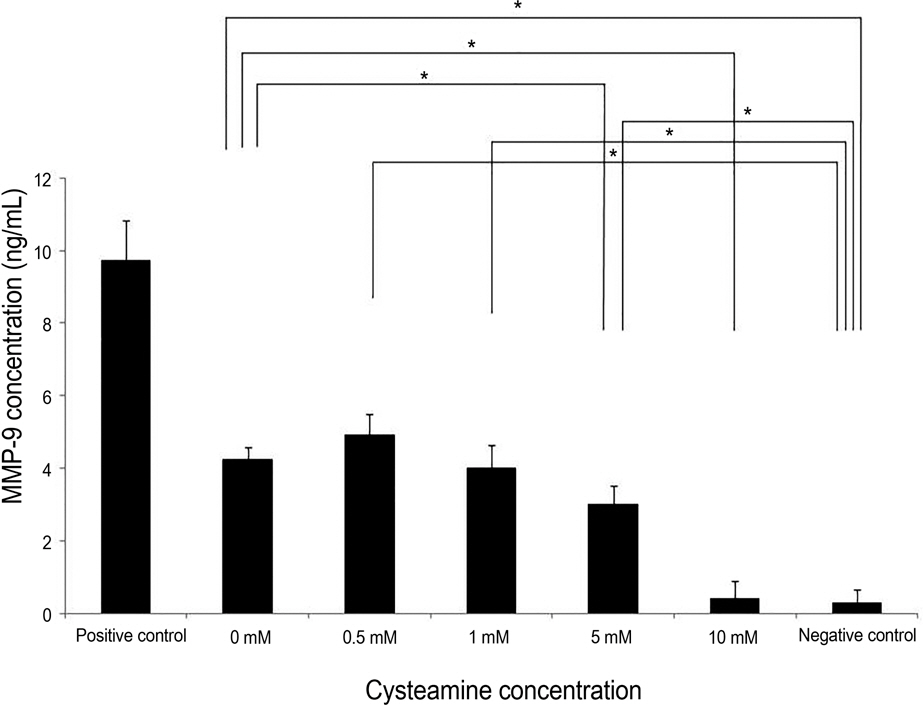
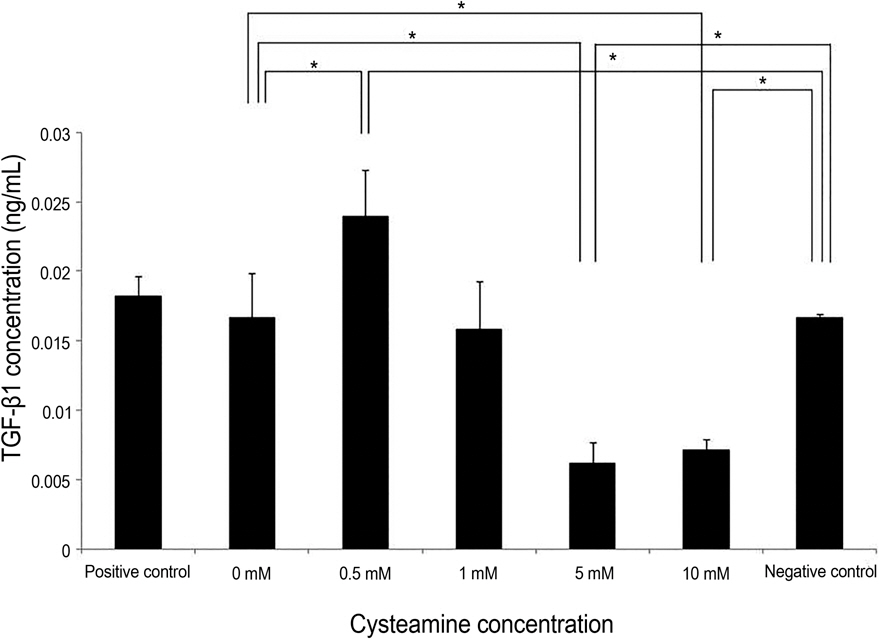
PBMC stimulation assay was performed after keratocytes were chemically injured with 0.05 N NaOH for 60 seconds. MLKR was treated with various concentrations of cysteamine (0-10 mM). Intracellular reactive oxygen species (ROS) formation was measured using the oxidation-sensitive fluorescent probe, 2'7'-dichlorofluorescein diacetate (DCF-DA). Proliferation rate of PBMCs stimulated by NaOH-treated keratocytes and secretion profiles of matrix metalloprotease-9 (MMP-9), transforming growth factor-beta1 (TGF-beta1), interleukin-6 (IL-6), and macrophage migration inhibitory factor (MIF) were determined using the bromodeoxyuridine proliferation assay and enzyme-linked immunosorbent assay, respectively.
RESULTS
Proliferation rate of PMBCs was suppressed by cysteamine in a dose-dependent manner (p = 0.019). Fluorescence of DCF-DA decreased depending on cysteamine concentration (p < 0.001). MMP-9, IL-6 and TGF-beta1 levels were suppressed by cysteamine in a dose-dependent manner (p < 0.05), whereas MIF levels increased with cysteamine concentration of 0.5-10 mM (p = 0.008).
CONCLUSIONS
These study results indicate that cysteamine induced the ROS-mediated inhibition of inflammatory cytokine release and proliferation of PBMCs stimulated by chemically injured keratocytes. Thus, cysteamine can be used in the treatment of chemical corneal burns.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997; 41:275–313.
Article2. Oh SY, Choi JS, Kim EJ. . The role of macrophage migration inhibitory factor in ocular surface disease pathogenesis after chem-ical burn in the murine eye. Mol Vis. 2010; 16:2402–11.3. Shi W, Liu J, Li M. . Expression of MMP, HPSE, and FAP in stroma promoted corneal neovascularization induced by different etiological factors. Curr Eye Res. 2010; 35:967–77.
Article4. Planck SR, Rich LF, Ansel JC. . Trauma and alkali burns in-duce distinct patterns of cytokine gene expression in the rat cornea. Ocul Immunol Inflamm. 1997; 5:95–100.
Article5. Gicquel JJ. Management of ocular surface chemical burns. Br J Ophthalmol. 2011; 95:159–61.
Article6. Chung JH, Kim HJ, Fagerholmb P, Cho BC. Effect of topically ap-plied Na-hyaluronan on experimental corneal alkali wound healing. Korean J Ophthalmol. 1996; 10:68–75.
Article7. Pattamatta U, Willcox M, Stapleton F, Garrett Q. Bovine lacto-ferrin promotes corneal wound healing and suppresses IL-1 ex-pression in alkali wounded mouse cornea. Curr Eye Res. 2013; 38:1110–7.
Article8. Dohlman CH, Cade F, Pfister R. Chemical burns to the eye: para-digm shifts in treatment. Cornea. 2011; 30:613–4.
Article9. Kubota M, Shimmura S, Kubota S. . Hydrogen and N-ace-tyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. 2011; 52:427–33.10. Kessler A, Biasibetti M, da Silva Melo DA. . Antioxidant ef-fect of cysteamine in brain cortex of young rats. Neurochem Res. 2008; 33:737–44.
Article11. Wilmer MJ, Kluijtmans LA, van der Velden TJ. . Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim Biophys Acta. 2011; 1812:643–51.
Article12. Choi YM, Park CG, Lee HS, Hur WJ. The effects of cysteamine on the radiation-induced apoptosis. J Korean Soc Ther Radiol Oncol. 2000; 18:214–19.13. Chakravarti S, Wu F, Vij N. . Microarray studies reveal macro-phage-like function of stromal keratocytes in the cornea. Invest Ophthalmol Vis Sci. 2004; 45:3475–84.
Article14. Funderburgh JL, Funderburgh ML, Mann MM. . Proteoglycan expression during transforming growth factor beta-induced kerato-cyte-myofibroblast transdifferentiation. J Biol Chem. 2001; 276:44173–8.15. Bourcier T, Borderie V, Forgez P. . In vitro effects of dex-amethasone on human corneal keratocytes. Invest Ophthalmol Vis Sci. 1999; 40:1061–70.16. Borderie VM, Lopez M, Lombet A. . Cryopreservation and culture of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1998; 39:1511–19.17. Musselmann K, Kane BP, Hassell JR. Isolation of a putative kera-tocyte activating factor from the corneal stroma. Exp Eye Res. 2003; 77:273–9.
Article18. Mosmann T. Rapid colorimetric assay for cellular growth and sur-vival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article19. Pathak S, Goldofsky E, Vivas EX. . IL-1β is overexpressed and aberrantly regulated in corticosteroid nonresponders with auto-immune inner ear disease. J Immunol. 2011; 186:1870–9.
Article20. Yang L, Hou Y. Different characters of spleen OX-62 positive den-dritic cells between Fischer and Lewis rats. Cell Mol Immunol. 2006; 3:145–50.21. Shin YJ, Kim JH, Seo JM. . Protective effect of clusterin on ox-idative stress-induced cell death of human corneal endothelial cells. Mol Vis. 2009; 15:2789–95.22. Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997; 41:275–313.
Article23. Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006; 28:219–42.
Article24. Saika S. TGF-beta signal transduction in corneal wound healing as a therapeutic target. Cornea. 2004; 23:(8 Suppl). S25–30.25. Jester JV, Barry PA, Lind GJ. . Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994; 35:730–43.26. Katakami C, Fujisawa K, Sahori A. . Localization of collagen (I) and collagenase mRNA by in situ hybridization during corneal wound healing after epikeratophakia or alkali-burn. Jpn J Ophthalmol. 1992; 36:10–22.27. Chakravarti S, Wu F, Vij N. . Microarray studies reveal macro-phage-like function of stromal keratocytes in the cornea. Invest Ophthalmol Vis Sci. 2004; 45:3475–84.
Article28. Bryan N, Ahswin H, Smart N. . Reactive oxygen species (ROS)-a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012; 24:249–65.29. Kovac S, Anqelova PR, Holmström KM. . Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015; 1850:794–801.
Article30. Misík V, Miyoshi N, Riesz P. Effects of cysteamine and cystamine on the sonochemical accumulation of hydrogen peroxide- im-plications for their mechanisms of action in ultrasound-exposed cells. Free Radic Biol Med. 1999; 26:961–7.31. Chen M, Matsuda H, Wang L. . Pretranscriptional regulation of Tgf-beta1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol Ther. 2010; 18:519–27.32. Saika S, Ikeda K, Yamanaka O. . Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol. 2005; 166:1405–18.
Article33. Zieske JD, Hutcheon AE, Guo X. . TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001; 42:1465–71.34. Sambursky R, O'Brien TP. MMP-9 and the perioperative manage-ment of LASIK surgery. Curr Opin Ophthalmol. 2011; 22:294–303.
Article35. Chang JH, Han KY, Azar DT. Wound healing fibroblasts modulate corneal angiogenic privilege: interplay of basic fibroblast growth factor and matrix metalloproteinases in corneal angiogenesis. Jpn J Ophthalmol. 2010; 54:199–205.
Article36. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003; 3:791–800.
Article37. Daun JM, Cannon JG. Macrophage migration inhibitory factor an-tagonizes hydrocortisone-induced increases in cytosolic IkappaBalpha. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R1043–49.38. Shin YJ, Seo JM, Chung TY. . Effect of cysteamine on oxida-tive stress-induced cell death of human corneal endothelial cells. Curr Eye Res. 2011; 36:910–7.
Article39. Waring GO. 3rd. Rodrigues MM. Patterns of pathologic response in the cornea. Surv Ophthalmol. 1987; 31:262–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of verapamil on cysteamine-induced duodenal ulcer in the rat
- The Effect of Cysteamine on the Radiation-Induced Apoptosis
- Mixed Mononuclear Cell Culture of Cord Blood and Adult Peripheral Blood
- Functional Changes of Interleukin-4 and Interferon-gamma of Peripheral Blood and Nasal Mucosa after Immunotherapy in Patients with Perennial Allergic Rhinitis
- The effects of silicone on mononuclear cell blastogenesis and il-1beta/tnf-alphasecretion of monocyte in human