J Korean Soc Radiol.
2012 Apr;66(4):375-384. 10.3348/jksr.2012.66.4.375.
Reversible Restricted Diffusion in the Corpus Callosum in Various Pediatric Diseases
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. hshong@schmc.ac.kr
- 2Department of Radiology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2208852
- DOI: http://doi.org/10.3348/jksr.2012.66.4.375
Abstract
- PURPOSE
To evaluate the reversible restricted diffusion in the corpus callosum in pediatric patients with clinical findings, and to discuss the possible pathogenesis of these lesions.
MATERIALS AND METHODS
Between 2007 and 2011, seven children with reversible signal abnormalities in the corpus callosum were identified and retrospectively reviewed.
RESULTS
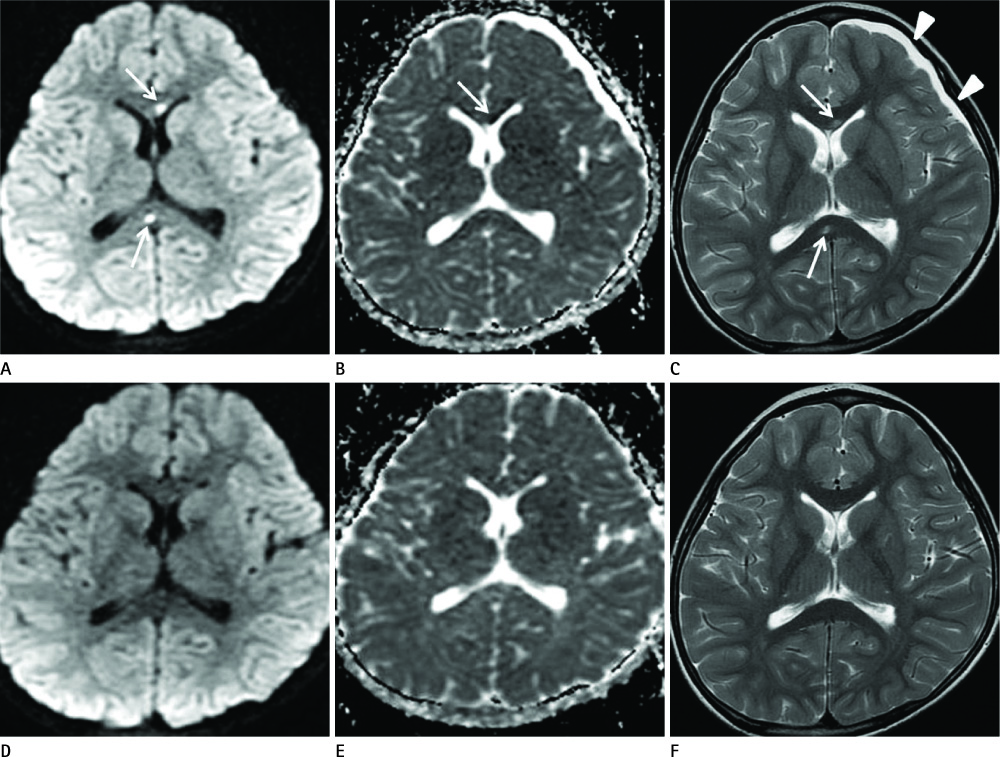
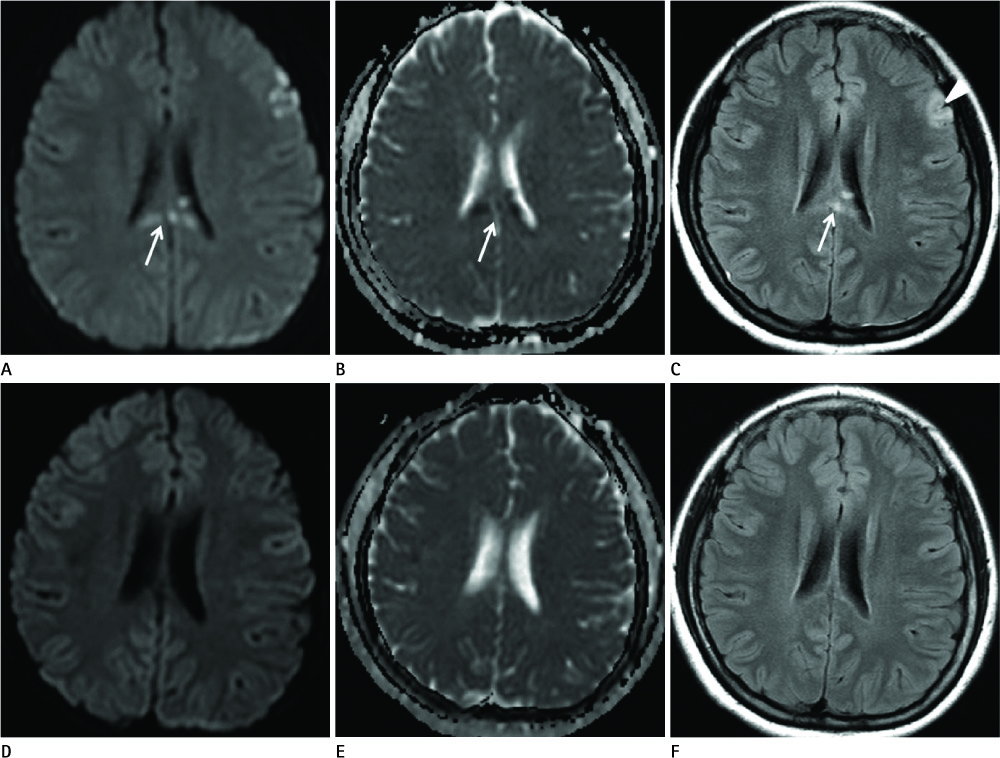
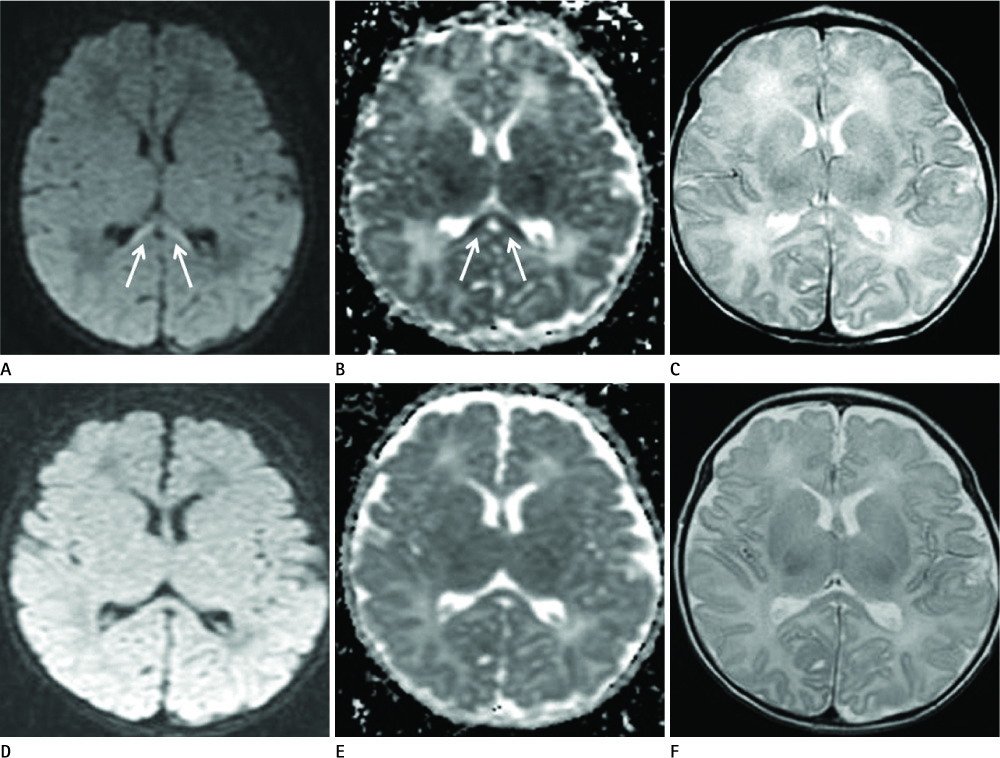
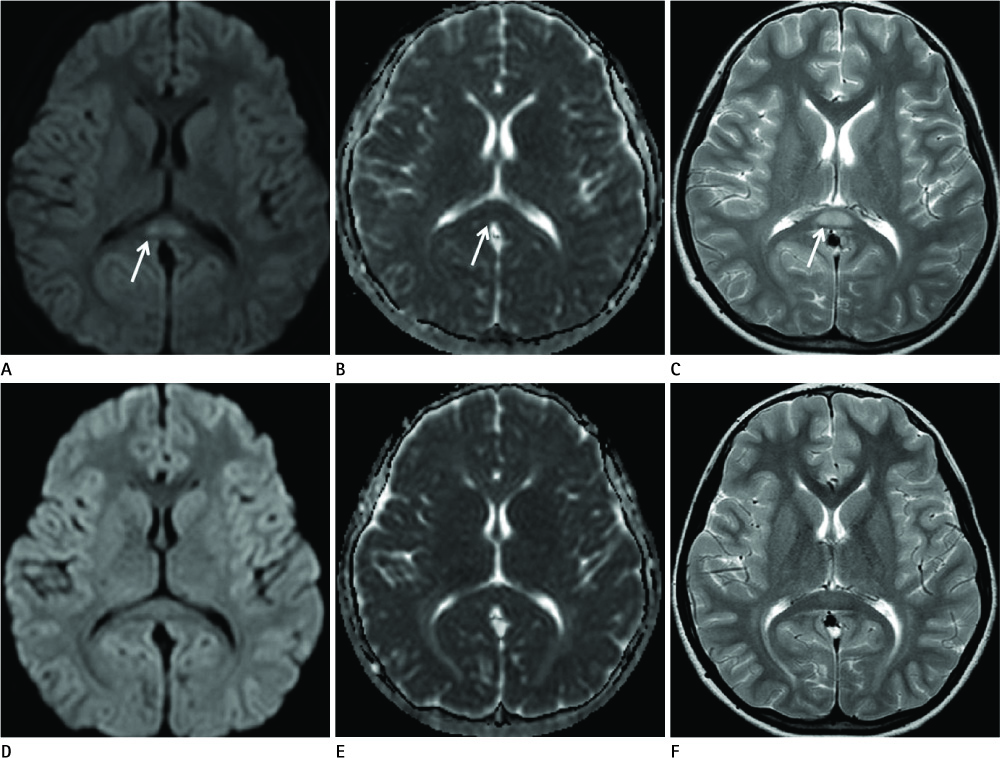
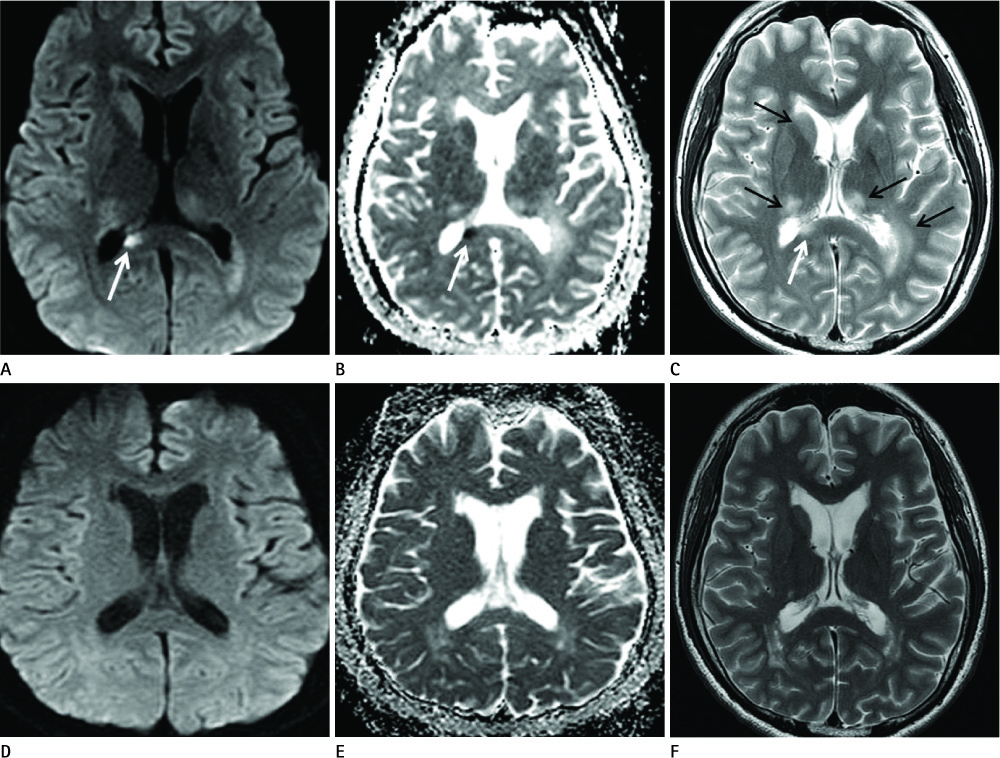
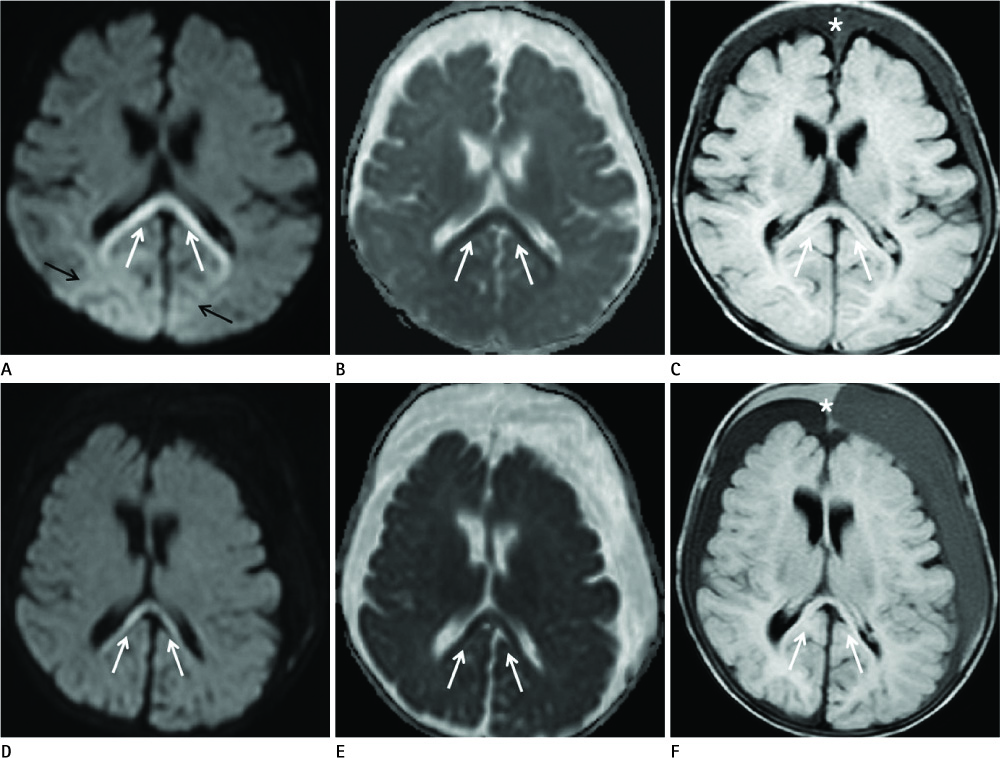
Diseases and conditions associated with lesions included: trauma (n = 3), neonatal seizure (n = 1), clinically suspected mild encephalopathy (n = 1), multiple sclerosis (n = 1), and seizure with subdural hygroma (n = 1). The callosal lesions were located in the splenium and the genu (n = 2), the splenium and the body (n = 1), and the splenium only (n = 4). The shape of the lesions was round-to-ovoid (n = 4) or linear (n = 3). Follow-up MRI scans showed completely resolved (n = 6) or persistent (n = 1) signal abnormalities on diffusion-weighted imaging as well as apparent diffusion coefficient mapping. Clinical outcomes were good in six of the patents but poor in the seventh.
CONCLUSION
Reversible restricted diffusion in the corpus callosum can develop in various diseases. Knowledge of the MRI findings and associated diseases might be helpful in predicting patients' conditions and clinical outcomes.
MeSH Terms
Figure
Reference
-
1. Matsukawa H, Shinoda M, Fujii M, Takahashi O, Yamamoto D, Murakata A, et al. Genu of corpus callosum in diffuse axonal injury induces a worse 1-year outcome in patients with traumatic brain injury. Acta Neurochir (Wien). 2011; 153:1687–1693. discussion 1693-16942. Kim SS, Chang KH, Kim ST, Suh DC, Cheon JE, Jeong SW, et al. Focal lesion in the splenium of the corpus callosum in epileptic patients: antiepileptic drug toxicity? AJNR Am J Neuroradiol. 1999; 20:125–129.3. Prilipko O, Delavelle J, Lazeyras F, Seeck M. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: report of two cases and literature review. Epilepsia. 2005; 46:1633–1636.4. Mirsattari SM, Lee DH, Jones MW, Blume WT. Transient lesion in the splenium of the corpus callosum in an epileptic patient. Neurology. 2003; 60:1838–1841.5. Matsubara K, Kodera M, Nigami H, Yura K, Fukaya T. Reversible splenial lesion in influenza virus encephalopathy. Pediatr Neurol. 2007; 37:431–434.6. Fukuda S, Kishi K, Yasuda K, Sejima H, Yamaguchi S. Rotavirus-associated encephalopathy with a reversible splenial lesion. Pediatr Neurol. 2009; 40:131–133.7. Ogura H, Takaoka M, Kishi M, Kimoto M, Shimazu T, Yoshioka T, et al. Reversible MR findings of hemolytic uremic syndrome with mild encephalopathy. AJNR Am J Neuroradiol. 1998; 19:1144–1145.8. Kim JH, Choi JY, Koh SB, Lee Y. Reversible splenial abnormality in hypoglycemic encephalopathy. Neuroradiology. 2007; 49:217–222.9. Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW, et al. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002; 179:251–257.10. Grühbaum B, Salzer H, Nasel C, Lernbass I. Reversible cytotoxic oedema in the splenium of the corpus callosum related to tetracycline therapy. Pediatr Radiol. 2010; 40:1693–1695.11. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004; 63:1854–1858.12. Karaarslan E, Arslan A. Diffusion weighted MR imaging in non-infarct lesions of the brain. Eur J Radiol. 2008; 65:402–416.13. Takanashi J, Maeda M, Hayashi M. Neonate showing reversible splenial lesion. Arch Neurol. 2005; 62:1481–1482. author reply 148214. Kubota T, Kidokoro H, Ito M, Oe H, Hattori T, Kato Y, et al. Diffusion-weighted imaging abnormalities in the corpus callosum after neonatal seizure: a case report. Brain Dev. 2008; 30:215–217.15. Takanashi J, Hirasawa K, Tada H. Reversible restricted diffusion of entire corpus callosum. J Neurol Sci. 2006; 247:101–104.16. Sigal T, Shmuel M, Mark D, Gil H, Anat A. Diffusion tensor imaging of corpus callosum integrity in multiple sclerosis: correlation with disease variables. J Neuroimaging. 2012; 22:33–37.17. Simon JH, Holtås SL, Schiffer RB, Rudick RA, Herndon RM, Kido DK, et al. Corpus callosum and subcallosal-periventricular lesions in multiple sclerosis: detection with MR. Radiology. 1986; 160:363–367.18. Maeda M, Tsukahara H, Terada H, Nakaji S, Nakamura H, Oba H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol. 2006; 33:229–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxic Brain Damage with Restricted Diffusion in the Splenium of Corpus Callosum
- Mild Encephalopathy with Reversible Lesion in the Splenium of the Corpus Callosum and Bilateral Frontal White Matter
- Lithium-Induced Downbeat Nystagmus with Reversible Splenial Lesion
- Recurrent Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion (MERS) on Diffusion Weighted Imaging: A Case Report
- Reversible Lesion in The splenium of The Corpus Callosum Induced by Topiramate in a Patient with Migraine