J Korean Soc Magn Reson Med.
2014 Dec;18(4):332-340. 10.13104/jksmrm.2014.18.4.332.
Clinical Utility of Prominent Hypointense Signals in the Draining Veins on Susceptibility-Weighted Imaging in Acute Cerebral Infarct: As a Marker of Penumbra and a Predictor of Prognosis
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ahn-kj@catholic.ac.kr
- 2Department of Neurology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- KMID: 2206930
- DOI: http://doi.org/10.13104/jksmrm.2014.18.4.332
Abstract
- PURPOSE
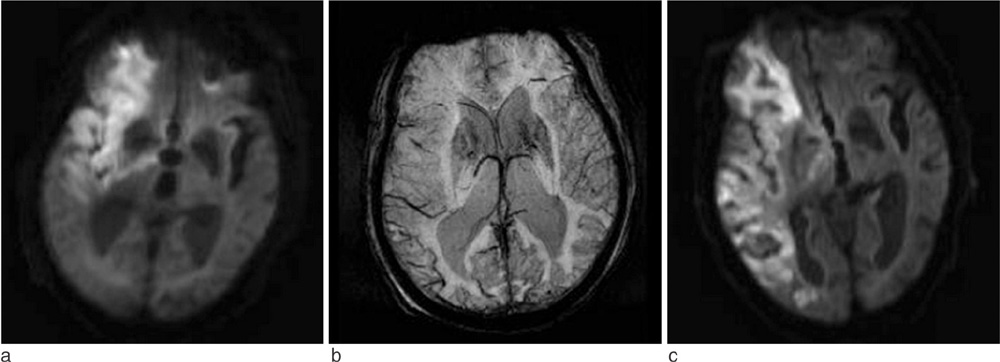
A relative increase in deoxyhemoglobin levels in hypoperfused tissue can cause prominent hypointense signals in the draining veins (PHSV) within areas of impaired perfusion in susceptibility-weighted imaging (SWI). The purpose of this study is to evaluate the usefulness of SWI in patients with acute cerebral infarction by evaluating PHSV within areas of impaired perfusion and to investigate the usefulness of PHSV in predicting prognosis of cerebral infarction.
MATERIALS AND METHODS
In 18 patients with acute cerebral infarction who underwent brain MRI with diffusion-weighted imaging and SWI and follow-up brain MRI or CT, we reviewed the presence and location of the PHSV within and adjacent to areas of cerebral infarction qualitatively and measured the signal intensity difference ratio of PHSVs to contralateral normal appearing cortical veins quantitatively on SWI. The relationship between the presence of the PHSV and the change in the extent of infarction in follow-up images was analyzed.
RESULTS
Of the 18 patients, 10 patients showed progression of the infarction, and 8 patients showed little change on follow-up imaging. On SWI, of the 10 patients with progression 9 patients showed peripheral PHSV and the newly developed infarctions corresponded well to area with peripheral PHSV on initial SWI. Only one patient without peripheral PHSV showed progression of the infarct. The patients with infarction progression revealed significantly higher presence of peripheral PHSV (p=0.0001) and higher mean signal intensity difference ratio (p=0.006) comparing to the patients with little change.
CONCLUSION
SWI can demonstrate a peripheral PHSV as a marker of penumbra and with this finding we can predict the prognosis of acute infarction. The signal intensity difference of PHSV to brain tissue on SWI can be used in predicting prognosis of acute cerebral infarction.
Keyword
MeSH Terms
Figure
Reference
-
1. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004; 52:612–618.2. Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008; 29:9–17.3. Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009; 64:74–83.4. Shen Y, Kou Z, Kreipke CW, Petrov T, Hu J, Haacke EM. In vivo measurement of tissue damage, oxygen saturation changes and blood flow changes after experimental traumatic brain injury in rats using susceptibility weighted imaging. Magn Reson Imaging. 2007; 25:219–227.5. Fujima N, Kudo K, Terae S, et al. Spinal arteriovenous malformation: evaluation of change in venous oxygenation with susceptibility-weighted MR imaging after treatment. Radiology. 2010; 254:891–899.6. Zaitsu Y, Kudo K, Terae S, et al. Mapping of cerebral oxygen extraction fraction changes with susceptibility-weighted phase imaging. Radiology. 2011; 261:930–936.7. Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke. 1995; 26:2272–2276.8. Stone SP, Allder SJ, Gladman JR. Predicting outcome in acute stroke. Br Med Bull. 2000; 56:486–494.9. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009; 30:19–30.10. Karaarslan E, Ulus S, Kurtuncu M. Susceptibility-weighted imaging in migraine with aura. AJNR Am J Neuroradiol. 2011; 32:E5–E7.11. Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981; 9:344–352.12. Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009; 30:232–252.13. Alexandrov AV, Black SE, Ehrlich LE, Caldwell CB, Norris JW. Predictors of hemorrhagic transformation occurring spontaneously and on anticoagulants in patients with acute ischemic stroke. Stroke. 1997; 28:1198–1202.14. Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intraarterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol. 2009; 30:165–170.15. Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007; 24:1–10.16. Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008; 39:2249–2256.17. Kassner A, Roberts TP, Moran B, Silver FL, Mikulis DJ. Recombinant tissue plasminogen activator increases blood-brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol. 2009; 30:1864–1869.18. Thornhill RE, Chen S, Rammo W, Mikulis DJ, Kassner A. Contrast-enhanced MR imaging in acute ischemic stroke: T2* measures of blood-brain barrier permeability and their relationship to T1 estimates and hemorrhagic transformation. AJNR Am J Neuroradiol. 2010; 31:1015–1022.19. Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990; 14:68–78.20. Forster BB, MacKay AL, Whittall KP, et al. Functional magnetic resonance imaging: the basics of blood-oxygen-level dependent (BOLD) imaging. Can Assoc Radiol J. 1998; 49:320–329.21. Powers WJ, Raichle ME. Positron emission tomography and its application to the study of cerebrovascular disease in man. Stroke. 1985; 16:361–376.22. Yamauchi H, Fukuyama H, Nagahama Y, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996; 61:18–25.23. He X, Zhu M, Yablonskiy DA. Validation of oxygen extraction fraction measurement by qBOLD technique. Magn Reson Med. 2008; 60:882–888.24. He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007; 57:115–126.25. An H, Lin W. Cerebral oxygen extraction fraction and cerebral venous blood volume measurements using MRI: effects of magnetic field variation. Magn Reson Med. 2002; 47:958–966.26. Haacke EM, Lai S, Reichenbach JR, et al. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: a direct validation of the blood oxygen level-dependent concept in functional brain imaging. Hum Brain Mapp. 1997; 5:341–346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypointense Rim on Susceptibility-Weighted Imaging in a Patient with Progressive Multifocal Leukoencephalopathy
- Acute Cerebral Infarction Associated With Stress Erythrocytosis: Susceptibility-Weighted Imaging Findings
- Susceptibility-Weighted MR Imaging for the Detection of Isolated Cortical Vein Thrombosis in a Patient with Spontaneous Intracranial Hypotension
- Gd-DTPA Eenhanced IVIRI of the Cerebral Venous Angiomas: Cornparision with Cerebral Angiography
- Quantitative Susceptibility Mapping of Oxygen Metabolism: A Feasibility Study Utilizing a Large-Scale Clinical Dataset