J Korean Neurosurg Soc.
2013 Dec;54(6):453-460. 10.3340/jkns.2013.54.6.453.
Changes of Motor Deactivation Regions in Patients with Intracranial Lesions
- Affiliations
-
- 1Department of Neurosurgery, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea. neurokoh@hanmail.net
- 2Department of Radiology, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 2190939
- DOI: http://doi.org/10.3340/jkns.2013.54.6.453
Abstract
OBJECTIVE
There is a rich literature confirming the default mode network found compatible with task-induced deactivation regions in normal subjects, but few investigations of alterations of the motor deactivation in patients with intracranial lesions. Therefore, we hypothesized that an intracranial lesion results in abnormal changes in a task-induced deactivation region compared with default mode network, and these changes are associated with specific attributes of allocated regions.
METHODS
Blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) during a motor task were obtained from 27 intracranial lesion patients (mean age, 57.3 years; range 15-78 years) who had various kinds of brain tumors. The BOLD fMRI data for each patient were evaluated to obtain activation or deactivation regions. The distinctive deactivation regions from intracranial lesion patients were evaluated by comparing to the literature reports.
RESULTS
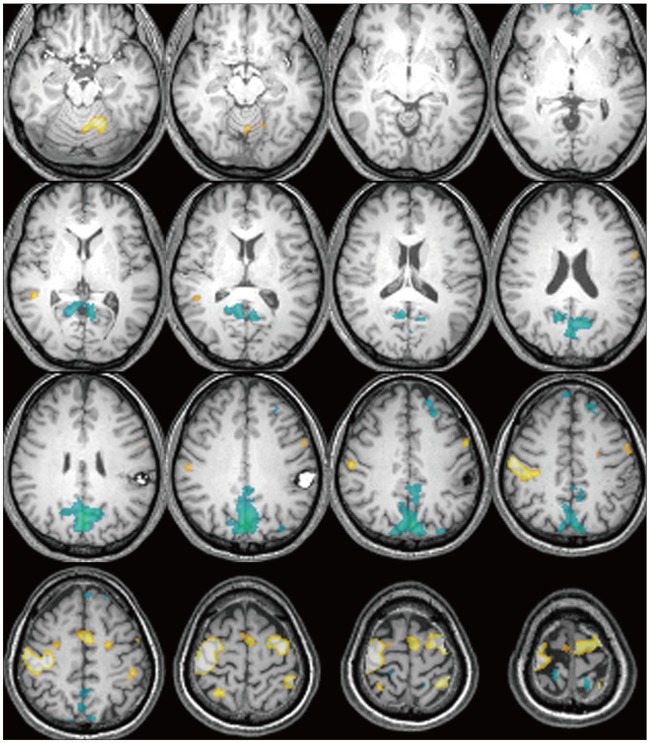
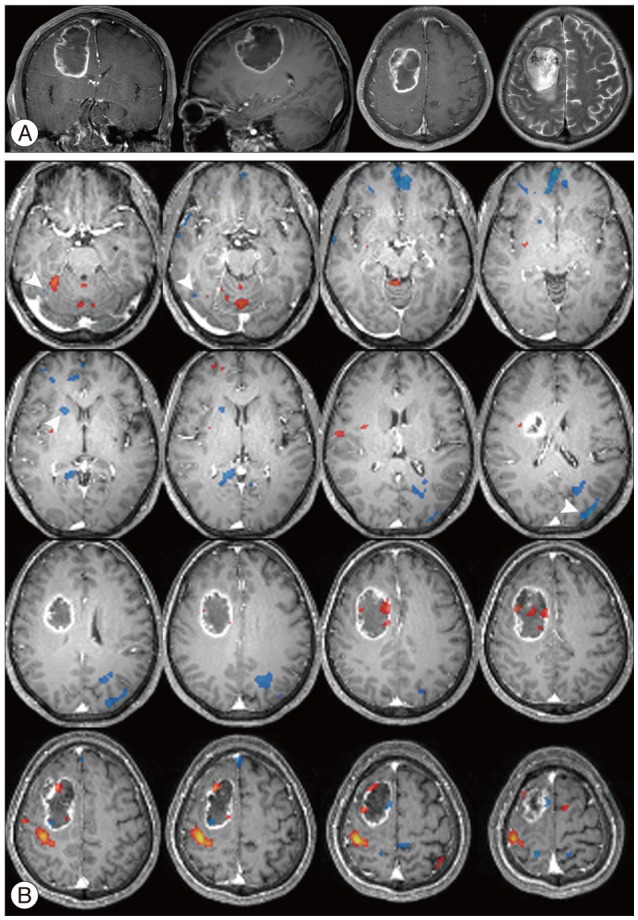
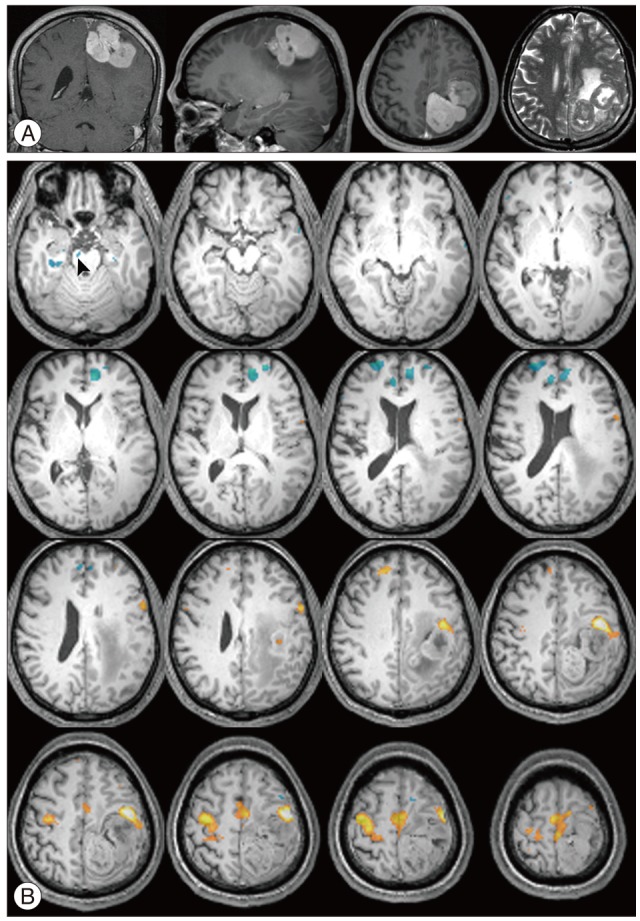
There were additive deactivated regions according to intracranial lesions: fusiform gyrus in cavernous hemangioma; lateral occipital gyrus in meningioma; crus cerebri in hemangiopericytoma; globus pallidus, lateral occipital gyrus, caudate nucleus, fusiform gyrus, lingual gyrus, claustrum, substantia nigra, subthalamic nucleus in GBM; fusiform gyrus in metastatic brain tumors.
CONCLUSION
There is increasing interest in human brain function using fMRI. The authors report the brain function migrations and changes that occur in patients with intracranial lesions.
MeSH Terms
Figure
Reference
-
1. Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000; 54:135–142. PMID: 10636139.
Article2. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005; 360:1001–1013. PMID: 16087444.
Article3. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995; 34:537–541. PMID: 8524021.
Article4. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008; 1124:1–38. PMID: 18400922.5. Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000; 21:1636–1644. PMID: 11039342.6. David N, Cohen MX, Newen A, Bewernick BH, Shah NJ, Fink GR, et al. The extrastriate cortex distinguishes between the consequences of one's own and others' behavior. Neuroimage. 2007; 36:1004–1014. PMID: 17478105.
Article7. Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001; 2:685–694. PMID: 11584306.
Article8. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002; 15:247–262. PMID: 11835612.
Article9. Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008; 44:936–952. PMID: 18635164.
Article10. Krüger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001; 46:631–637. PMID: 11590638.
Article11. Marchand WR, Lee JN, Thatcher JW, Thatcher GW, Jensen C, Starr J. Motor deactivation in the human cortex and basal ganglia. Neuroimage. 2007; 38:538–548. PMID: 17888686.
Article12. Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010; 46:691–699. PMID: 19775684.
Article13. Mayer JS, Roebroeck A, Maurer K, Linden DE. Specialization in the default mode: Task-induced brain deactivations dissociate between visual working memory and attention. Hum Brain Mapp. 2010; 31:126–139. PMID: 19639552.
Article14. Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001; 54:287–298. PMID: 11287133.
Article15. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:676–682. PMID: 11209064.
Article16. Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011; 134(Pt 8):2233–2247. PMID: 21841202.17. Villablanca JR. Why do we have a caudate nucleus? Acta Neurobiol Exp (Wars). 2010; 70:95–105. PMID: 20407491.