J Korean Assoc Oral Maxillofac Surg.
2012 Apr;38(2):121-126. 10.5125/jkaoms.2012.38.2.121.
Genetic aberrations on the short arm of chromosome 8 (8p) in tongue carcinomas
- Affiliations
-
- 1Department of Dentistry and Oral Surgery, Chiba University Hospital, Chiba, Japan. murano-cib@umin.ac.jp
- KMID: 2189713
- DOI: http://doi.org/10.5125/jkaoms.2012.38.2.121
Abstract
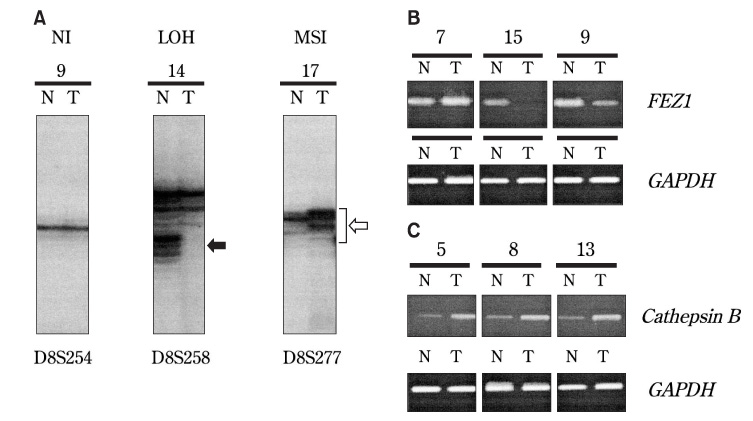
- Aberrations on the short arm of chromosome 8 (8p) are frequently observed in several human cancers. In this study, 20 squamous cell carcinoma (SCC) specimens from the tongue were examined in order to evaluate the role of 8p in SCC of the tongue. Microsatellite analysis using 14 markers demonstrated two commonly deleted regions (CDRs) on 8p. Reverse transcription-polymerase chain reaction (RT-PCR) revealed frequent down-regulation of the FEZ1 gene, mapped to 8p22, and frequent over-expression of the cathepsin B gene, mapped to 8p-21-22. These results suggested that genetic aberrations are involved in the development of SCC of the tongue. However, no significant relationship was observed to be established between the genetic alterations and clinicopathological features. Thus, further investigation is necessary in order to clarify the clinical role of 8p in carcinoma of the tongue.
MeSH Terms
Figure
Reference
-
1. Nowell PC, Croce CM. Chromosomes, genes, and cancer. Am J Pathol. 1986. 125:7–15.
Article2. Nowell PC. Foundations in cancer research. Chromosomes and cancer: the evolution of an idea. Adv Cancer Res. 1993. 62:1–17.
Article3. Knudson AG. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993. 90:10914–10921.
Article4. Suzuki H, Emi M, Komiya A, Fujiwara Y, Yatani R, Nakamura Y, et al. Localization of a tumor suppressor gene associated with progression of human prostate cancer within a 1.2 Mb region of 8p22-p21.3. Genes Chromosomes Cancer. 1995. 13:168–174.
Article5. Cunningham C, Dunlop MG, Bird CC, Wyllie AH. Deletion analysis of chromosome 8p in sporadic colorectal adenomas. Br J Cancer. 1994. 70:18–20.
Article6. Kerangueven F, Essioux L, Dib A, Noguchi T, Allione F, Geneix J, et al. Loss of heterozygosity and linkage analysis in breast carcinoma: indication for a putative third susceptibility gene on the short arm of chromosome 8. Oncogene. 1995. 10:1023–1026.7. Knowles MA, Shaw ME, Proctor AJ. Deletion mapping of chromosome 8 in cancers of the urinary bladder using restriction fragment length polymorphisms and microsatellite polymorphisms. Oncogene. 1993. 8:1357–1364.8. el-Naggar AK, Hurr K, Batsakis JG, Luna MA, Goepfert H, Huff V. Sequential loss of heterozygosity at microsatellite motifs in preinvasive and invasive head and neck squamous carcinoma. Cancer Res. 1995. 55:2656–2659.9. Wu CL, Roz L, Sloan P, Read AP, Holland S, Porter S, et al. Deletion mapping defines three discrete areas of allelic imbalance on chromosome arm 8p in oral and oropharyngeal squamous cell carcinomas. Genes Chromosomes Cancer. 1997. 20:347–353.
Article10. Ishii H, Baffa R, Numata SI, Murakumo Y, Rattan S, Inoue H, et al. The FEZ1 gene at chromosome 8p22 encodes a leucine-zipper protein, and its expression is altered in multiple human tumors. Proc Natl Acad Sci U S A. 1999. 96:3928–3933.
Article11. Ono K, Uzawa K, Nakatsuru M, Shiiba M, Mochida Y, Tada A, et al. Down-regulation of FEZ1/LZTS1 gene with frequent loss of heterozygosity in oral squamous cell carcinomas. Int J Oncol. 2003. 23:297–302.
Article12. Wahi PN. World Health Organization. Histological typing of oral and orophangeal tumors. International histological classification of tumours. 1971. vol. 4. Geneva: World Health Organization.13. Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988. 61:2310–2314.
Article14. Maniatis T, Fritsch EF, Sambrook J. Molecular cloning, a laboratory manual. 1982. 1st ed. New York: Cold Spring Harbor;280–281.15. Ono K, Miyakawa A, Fukuda M, Shiiba M, Uzawa K, Watanabe T, et al. Allelic loss on the short arm of chromosome 8 in oral squamous cell carcinoma. Oncol Rep. 1999. 6:785–789.
Article16. Takechi T, Okabe H, Fujioka A, Murakami Y, Fukushima M. Relationship between protein levels and gene expression of dihydropyrimidine dehydrogenase in human tumor cells during growth in culture and in nude mice. Jpn J Cancer Res. 1998. 89:1144–1153.
Article17. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000. 36:256–263.
Article18. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncol. 2000. 36:311–327.
Article19. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 3: clinico-pathological applications. Oral Oncol. 2000. 36:404–413.
Article20. Partridge M, Emilion G, Pateromichelakis S, Phillips E, Langdon J. Location of candidate tumour suppressor gene loci at chromosomes 3p, 8p and 9p for oral squamous cell carcinomas. Int J Cancer. 1999. 83:318–325.
Article21. Vecchione A, Ishii H, Shiao YH, Trapasso F, Rugge M, Tamburrino JF, et al. Fez1/lzts1 alterations in gastric carcinoma. Clin Cancer Res. 2001. 7:1546–1552.22. Kawaki J, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, et al. Allelic loss in human intrahepatic cholangiocarcinoma: correlation between chromosome 8p22 and tumor progression. Int J Cancer. 2000. 88:228–231.
Article23. Mort JS, Buttle DJ. Cathepsin B. Int J Biochem Cell Biol. 1997. 29:715–720.
Article24. Podgorski I, Sloane BF. Cathepsin B and its role (s) in cancer progression. Proteases and the regulation of biological processes. Proceedings of the Biochemical Society Symposium. 2003. vol. 70. London: Portland Press;263–276.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prenatal Diagnosis of a Satellited Chromosome 8p Results from a de novo Cryptic Translocation between Chromosomes 8 and 22
- Chromosomal Aberrations in Korean Hepatocellular Carcinomas
- A Case of Partial Short Arm Deletion in Chromosome 9 with Inguinal Hernia, Testicular Cystic Lesion, and Arthrogryposis Multiplex Congenita
- A Case of Partial Monosomy lOq Syndrome
- The general anesthesia experience of deletion 8p syndrome patient: A case report