J Korean Med Assoc.
2009 Nov;52(11):1059-1068. 10.5124/jkma.2009.52.11.1059.
Novel Pharmacotherapies for Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Konkuk University School of Medicine Center for Geriatric Neuroscience Research, IBST, Konkuk University, Korea. alzdoc@kuh.ac.kr
- KMID: 2188088
- DOI: http://doi.org/10.5124/jkma.2009.52.11.1059
Abstract
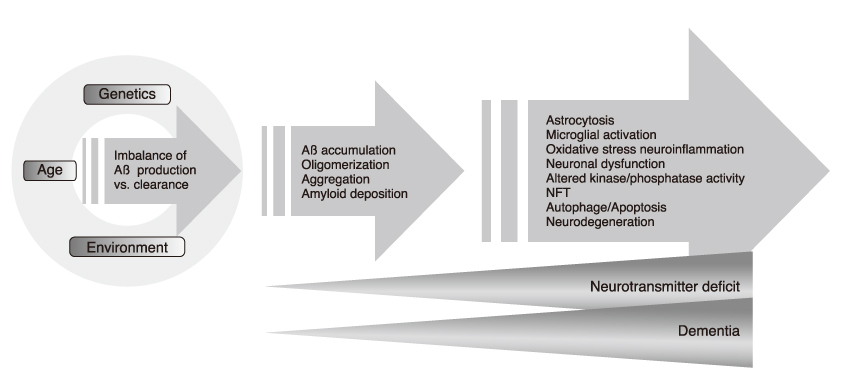
- Alzheimer disease (AD) is pathologically characterized by extracellular amyloid deposits composed of beta-amyloid (A beta) peptide, neurofibrillary tangles (NFTs) made up of hyperphosphorylated tau, and deficit of cholinergic neurons in the basal forebrain. It is the most common neurodegenerative disease in the elderly. With the aging of the population, the incidence and prevalence of AD will also increase rapidly. The subsequent growing socioeconomic burden seems to be inevitable until effective therapeutic strategies are developed. Currently available treatments approved by the US Food and Drug Administration, while ameliorating the symptoms, do not halt progression or cure the illness. AD is a multifactorial syndrome with several target proteins contributing to its etiology. In this review, various small molecules targeting pathological hall marks or their major constituents that have been reported in the literature will be discussed, with emphasis on compounds that are presently being investigated in clinical trials.
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacokinetics, Pharmacodynamics and Safety of JES9501 after Single and Multiple Oral Administration in Healthy Subjects
AnHye Kim, Bo-Hyung Kim, Dongseong Shin, Joo-Youn Cho, Kyung-Sang Yu, In-Jin Jang, Jae-Yong Chung
J Korean Soc Clin Pharmacol Ther. 2013;21(2):141-149. doi: 10.12793/ikscpt.2013.21.2.141.
Reference
-
1. Vetrivel KS, Thinakaran G. Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments. Neurology. 2006. 66:S69–S73.2. Lee VM, Trojanowski JQ. Mechanisms of parkinson's disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006. 52:33–38.
Article3. Roberson MR, Harrell LE. Cholinergic activity and amyloid precursor protein metabolism. Brain Res Brain Res Rev. 1997. 25:50–69.
Article4. Roberson MR, Kolasa K, Parsons DS, Harrell LE. Cholinergic denervation and sympathetic ingrowth result in persistent changes in hippocampal muscarinic receptors. Neuroscience. 1997. 80:413–418.
Article5. Bartolini M, Bertucci C, Cavrini V, Andrisano V. Beta-amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem Pharmacol. 2003. 65:407–416.
Article6. De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry. 2001. 40:10447–10457.
Article7. Hardy J. Molecular genetics of alzheimer's disease. Acta Neurol Scand Suppl. 1996. 165:13–17.
Article8. Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial alzheimer's disease. Nature. 1995. 375:754–760.
Article9. Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Familial alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the alzheimer's disease type 3 gene. Nature. 1995. 376:775–778.
Article10. Hardy J. Alzheimer's disease: The amyloid cascade hypothesis: An update and reappraisal. J Alzheimers Dis. 2006. 9:151–153.
Article11. Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008. 14:837–842.
Article12. Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002. 416:535–539.
Article13. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002. 298:789–791.
Article14. Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005. 25:7709–7717.
Article15. Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in alzheimer disease. Arch Neurol. 2002. 59:1381–1389.
Article16. Holtzman DM. Amyloid-beta binding molecule: Potential role in the pathogenesis and treatment of alzheimer disease. Alzheimer Dis Assoc Disord. 2003. 17:Suppl 2. S66–S68.17. Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of alzheimer disease and relation to apolipoprotein e genotype. Arch Neurol. 1998. 55:937–945.
Article18. Asai M, Hattori C, Iwata N, Saido TC, Sasagawa N, Szabo B, Hashimoto Y, Maruyama K, Tanuma S, Kiso Y, Ishiura S. The novel beta-secretase inhibitor kmi-429 reduces amyloid beta peptide production in amyloid precursor protein transgenic and wild-type mice. J Neurochem. 2006. 96:533–540.
Article19. Stachel SJ. Progress toward the development of a viable BACE-1 inhibitor. Drug Develop Research. 2009. 70:101–110.
Article20. Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003. 5:486–488.21. Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and cns defects in presenilin-1-deficient mice. Cell. 1997. 89:629–639.
Article22. Wu WL, Zhang L. Gamma-secretase Inhibitors for the treatment of Alzheimer's disease. Drug Develop Resear. 2000. 70:94–100.23. Bell KF, Claudio Cuello A. Altered synaptic function in alzheimer's disease. Eur J Pharmacol. 2006. 545:11–21.
Article24. Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007. 51:92–104.
Article25. Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic abeta42 by inhibiting rho. Science. 2003. 302:1215–1217.
Article26. Tjernberg LO, Naslund J, Lindqvist F, Johansson J, Karlstrom AR, Thyberg J, Terenius L, Nordstedt C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J Biol Chem. 1996. 271:8545–8548.27. Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castano EM, Frangione B. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for alzheimer's therapy. Nat Med. 1998. 4:822–826.
Article28. Hughes E, Burke RM, Doig AJ. Inhibition of toxicity in the beta-amyloid peptide fragment beta-(25-35) using n-methylated derivatives: A general strategy to prevent amyloid formation. J Biol Chem. 2000. 275:25109–25115.
Article29. Bush AI. The metallobiology of alzheimer's disease. Trends Neurosci. 2003. 26:207–214.
Article30. White AR, Du T, Laughton KM, Volitakis I, Sharples RA, Xilinas ME, Hoke DE, Holsinger RM, Evin G, Cherny RA, Hill AF, Barnham KJ, Li QX, Bush AI, Masters CL. Degradation of the alzheimer disease amyloid beta-peptide by metal-dependent up-regulation of metalloprotease activity. J Biol Chem. 2006. 281:17670–17680.
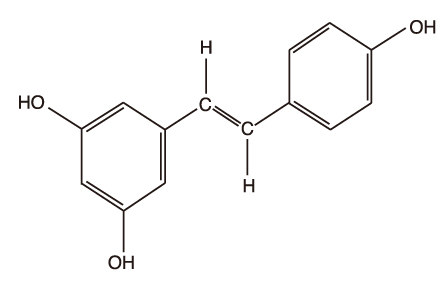
Article31. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur J Pharmacol. 2006. 545:51–64.
Article32. Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001. 22:993–1005.
Article33. Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat Med. 2003. 9:448–452.
Article34. Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of abeta42 immunisation in alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008. 372:216–223.
Article35. Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M. Long-term follow-up of patients immunized with an1792: Reduced functional decline in antibody responders. Curr Alzheimer Res. 2009. 6:144–151.
Article36. Relkin NR. Beyond symptomatic therapy: A re-examination of acetylcholinesterase inhibitors in alzheimer's disease. Expert Rev Neurother. 2007. 7:735–748.
Article37. Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves rage and pecam-1. Am J Physiol Cell Physiol. 2000. 279:C1772–C1781.38. Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of alzheimer's amyloid-ss (1-40) peptide from brain by ldl receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000. 106:1489–1499.
Article39. Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007. 13:1029–1031.
Article40. Guan H, Liu Y, Daily A, Police S, Kim MH, Oddo S, LaFerla FM, Pauly JR, Murphy MP, Hersh LB. Peripherally expressed neprilysin reduces brain amyloid burden: A novel approach fortreating alzheimer's disease. J Neurosci Res. 2009. 87:1462–1473.
Article41. Farris W, Mansourian S, Leissring MA, Eckman EA, Bertram L, Eckman CB, Tanzi RE, Selkoe DJ. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol. 2004. 164:1425–1434.
Article42. Nalivaeva NN, Fisk LR, Belyaev ND, Turner AJ. Amyloid-degrading enzymes as therapeutic targets in alzheimer's disease. Curr Alzheimer Res. 2008. 5:212–224.
Article43. Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia ftdp-17. Nature. 1998. 393:702–705.
Article44. Savage MJ, Gingrich DE. Advances in the development of kinase inhibitor therapeutics for Alzheimer's disease. Drug Deveop Res. 2009. 70:125–144.
Article45. Sigurdsson EM. Immunotherapy targeting pathological tau protein in alzheimer's disease and related tauopathies. J Alzheimers Dis. 2008. 15:157–168.
Article46. Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate alzheimer's disease: A randomised, double-blind, placebo-controlled study. Lancet. 2008. 372:207–215.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Memorials of Alois Alzheimer (June 14, 1864~December 19, 1915) and Historical Background of Alzheimer's Disease
- Significance of Non-Alzheimer Dementia
- Current Diagnostic Criteria of Alzheimer's Disease
- Pathobiolgy and Management of Alzheimer’s Disease
- Autopy Results of Clinically Diagnosed Alzheimer's Disease