J Korean Orthop Assoc.
2009 Feb;44(1):37-45. 10.4055/jkoa.2009.44.1.37.
Age Related Local Growth Factors Affect Muscle Regeneration in Distraction Osteogenesis
- Affiliations
-
- 1Department of Orthopedic Surgery, Chungnam National University School of Medicine, Daejeon, Korea. hyunsd@cnu.ac.kr
- KMID: 2186376
- DOI: http://doi.org/10.4055/jkoa.2009.44.1.37
Abstract
-
PURPOSE: To evaluate the effect of growth factors on muscle regeneration related to the distraction rate and age on bone distraction.
MATERIALS AND METHODS
This study examined the effects in the tibialis anterior (TA) and soleus muscles after tibial bone distraction in 6 young and mature rats. The young and old rats were 6 weeks old (average 250 mg) and 6 months old (average 450 mg), respectively. In all rats, the right tibial bone was distracted, and the left was used as a control group. The development pattern of IGF-1, PDGF, bFGF was assessed using immunohistochemical techniques. The distraction rate was 0.7 mm/day (2 times) and the total extension ratio was 20% in all cases.
RESULTS
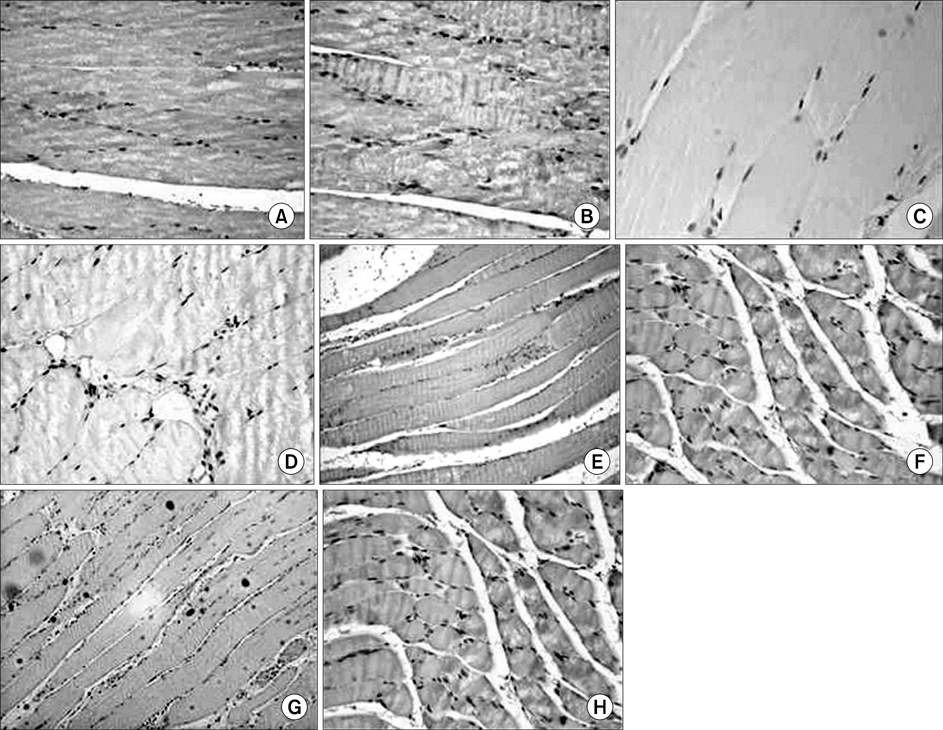
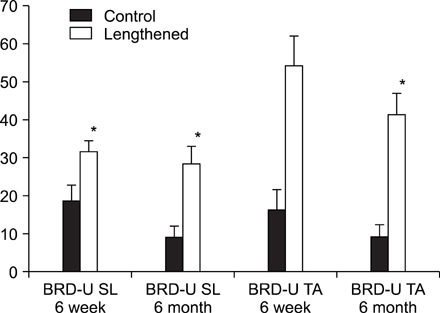
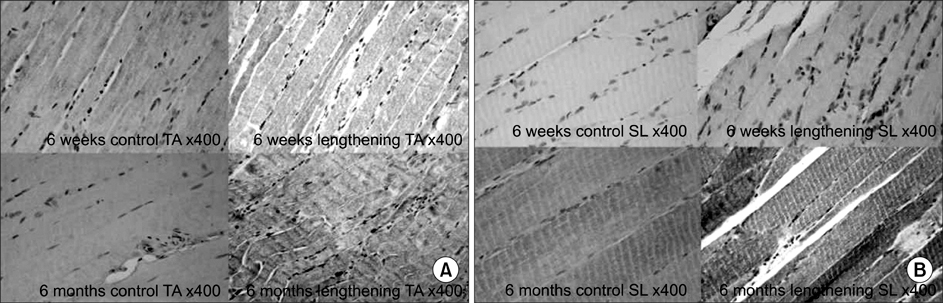
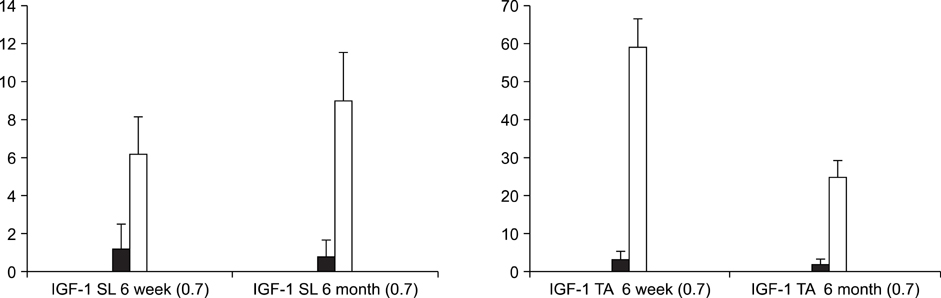
In the TA muscle, the development of IGF-1 and PDGF was inversely proportional to age (p<0.05). In the soleus muscle, the development of IGF-1 and PDGF was higher in the old rats but the difference was not statistically significant. BrdU positive cells were expressed in the myosatellite cells and the basement membrane of myocytes. IGF-1 and PDGF was also expressed in myosatellite cells but bFGF was not. bFGF facilitated bone regeneration but the mechanism for its effects on muscle regeneration were not identified.
CONCLUSION
Distraction osteogenesis facilitates the release of growth factors. Immature muscles adapt to the distraction but mature muscles do not. This suggests that mature muscles are less able to activate the proliferation and differentiation of myosatellite cells and the local release of growth factors when bone distraction is performed.
MeSH Terms
Figure
Reference
-
1. Anderson JE, Liu L, Kardami E. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev Biol. 1991. 147:96–109.
Article2. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998. 95:15603–15607.
Article3. Bischoff R. Engel AG, Franzini-Armstrong C, editors. The satellite cell and muscle regeneration. Myology: basic and clinical. 1994. New York, etc.: McGraw Hill;97–118.4. Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol. 1990. 258:C436–C442.
Article5. Cannon JG. Intrinsic and extrinsic factors in muscle aging. Ann NY Acad Sci. 1992. 854:72–77.
Article6. Carlson BM. Muscle regeneration and aging. Monogr Dev Biol. 1992. 23:189–195.7. Carson JA, Yan Z, Booth FW, Coleman ME, Schwartz RJ, Stump CS. Regulation of skeletal alpha-actin promotor in young chickens during hypertrophy caused by stretch overload. Am J Physiol. 1995. 268:C918–C924.8. Chakravarthy MV, Davis BS, Booth FW. IGF-1 restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000. 89:1365–1379.9. Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Can J Appl Physiol. 1996. 21:155–184.
Article10. Codivilla A. On the means of the lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1904. Clin Orthop Relat Res. 1994. 301:4–9.11. Day CS, Moreland MS, Floyd SS Jr, Huard J. Limb lengthening promotes muscle growth. J Orthop Res. 1997. 15:227–234.
Article12. De Deyne PG, Meyer R, Paley D, Herzenberg JE. The adaptation of perimuscular connective tissue during distraction osteogenesis. Clin Orthop Relat Res. 2000. 379:259–269.
Article13. Dix DJ, Eisenberg BR. Myosin mRNA accumulation and myofibrillogenersis at the myotendinous junction of stretched muscle fibers. J Cell Biol. 1990. 111:1885–1894.14. Florini JR, Magri KA. Effect of growth factors on myogenic differentiation. Am J Physiol. 1989. 256:C701–C711.15. Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol. 1992. 262:R356–R363.
Article16. Goldspink G, Yang SY, Skarli M, Vrbova G. Local growth regulation is associated with an IGF-1 isoform og IGF-1 that is expressed in normal muscle but not in dystrophic mdx or dydy mouse muscle when subjected to stretch. J Physiol. 1996. 495:162–163.17. Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998. 854:78–91.18. Grounds MD. Towards understanding skeletal muscle regeneration. Pathol Res Pract. 1991. 187:1–22.
Article19. Haddad F, Adams GR. The time course of cellular and molecular responses to a single bout of resistance exercise in rodent skeletal muscle. 2001. In : IUPS Conference; New Zealand.20. Hawke TJ, Jiang N, Garry DJ. Absence of p21CIP rescues myogenic progenitor cell proliferative and regenerative capacity in Foxk1 null mice. J Biol Chem. 2003. 278:4015–4020.
Article21. Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc. 1992. 24:197–205.
Article22. Jennische E. Sequential immunohistochemical expression of IGF-1 and the transferrin receptor in regenerating rat muscle in vivo. Acta Endocriol (Copenh). 1989. 121:733–738.23. Jennische E, Hansson HA. Regenerating skeletal muscle cells express insulin-like growth factor 1. Acta Physiol Scand. 1987. 130:327–332.24. Jin P, Rahm M, Claesson-Welsh L, Heldin CH, Sejerson T. Expression of PDGF A chain and beta-receptor genes during rat myoblast differentiation. J Cell Biol. 1990. 110:1665–1672.25. Johnson SE, Allen RE. Activation of skeletal muscle satellite cells and the role of fibroblast growth factor receptors. Exp Cell Res. 1995. 219:449–453.
Article26. Lagord C, Carpentier G, Leibovitch MP, Gautron J, Martelly I. Stimulation of rat satellite cell myogenersis by inhibitors of ser/thr protein kinases. Neuromolecul Disord. 1993. 3:379–383.27. Lefaucheur JP, Sébille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic-fibroblast growth factor, transforming growth factor Pi or insulin-like growth factor 1. J Neuroimmunol. 1995. 57:85–91.28. Li Xun, Kim KC, Choi JH, Lee HJ, Shin HD. Effect of local growth factors on muscle regeneration in distraction osteogenesis. J Korean Orthop Assoc. 2006. 41:1028–1036.
Article29. Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1998. 333:560–562.30. Pattison JS, Folk LC, Madsen RW, Childs TE, Booth FW. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics. 2003. 15:34–43.
Article31. Tidball JG, Spencer MJ. PDGF stimulation induces phosphorylation of talin and cytoskeletal reorganization in skeletal muscle. J Cell Biol. 1993. 123:627–635.
Article32. Wiedenman JL, Rivera-Rivera I, Vyas D, et al. Beta-MHC and SMLC1 transgene induction in overloaded skeletal muscle of transgene mice. Am J Physiol. 1996. 270:C1111–C1121.33. Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol. 1990. 111:1623–1629.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Local Growth Factors on Muscle Regeneration in Distraction Osteogenesis
- Case reports of antero-posteior movement with distraction osteogenesis in maxillary anterior segment
- Role of Angiogenic Stem Cells in Bone Regeneration
- The histological and immunohistochemical findings of the newly formed human bone after distraction osteogenesis
- Maxillary distraction osteogenesis in the management of cleft lip and palate: report of 2 cases