J Korean Med Assoc.
2006 Feb;49(2):163-172. 10.5124/jkma.2006.49.2.163.
Clinical Application of Nutrigenomics
- Affiliations
-
- 1Department of Gastroenterology/Genome Research Center for Gastroenterology, Ajou University College of Medicine and Hospital, Korea. ms8686@hanmail.net, hahmkb@hotmail.com
- 2Postgraduate School of Public Health and Institute of Health and Environment, Seoul National University, Korea. hjjoung@snu.ac.kr
- KMID: 2184664
- DOI: http://doi.org/10.5124/jkma.2006.49.2.163
Abstract
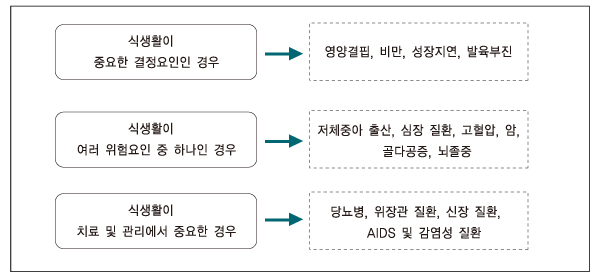
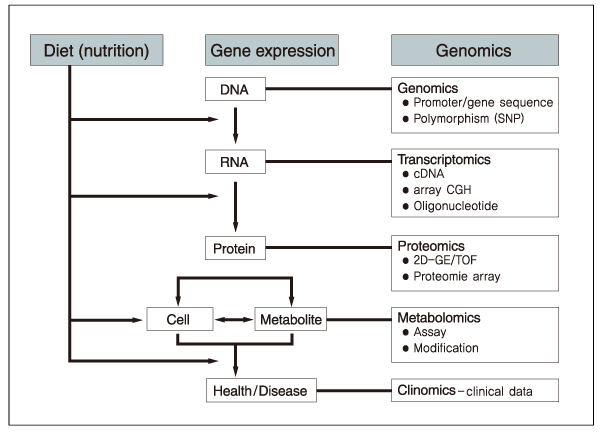
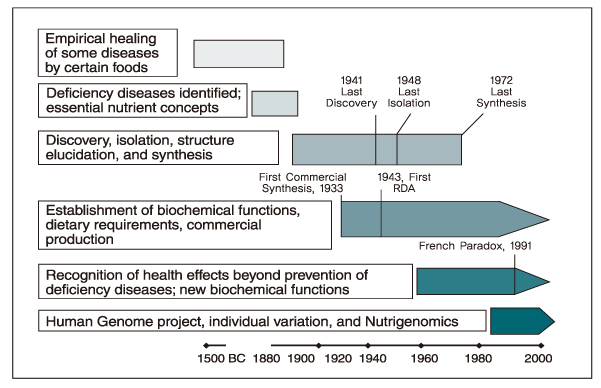
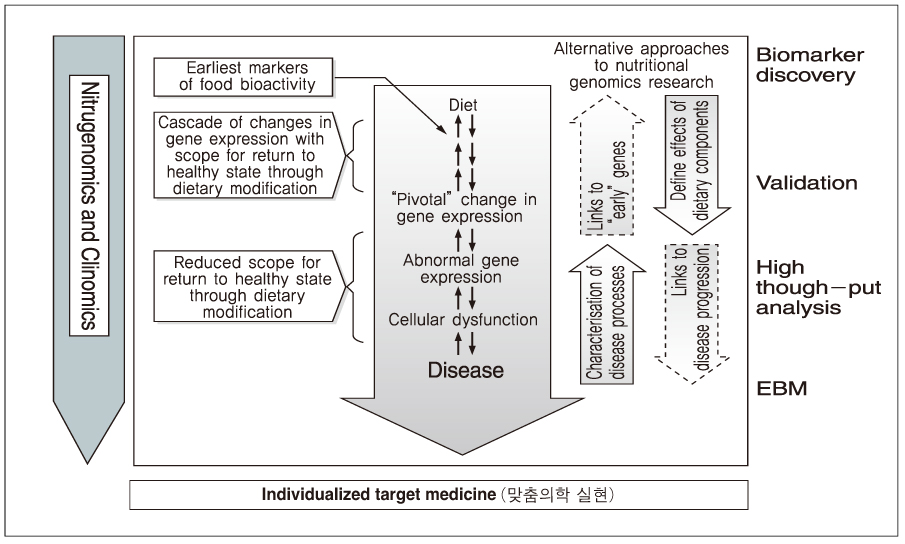
- Nutritional genomics (nutrigenomics) is the application of high-throughput functional genomics technologies to nutritional science lying in the interface between the nutritional environment and genetic process. It seeks to provide a molecular genetic understanding of how common dietary nutrition affects health by altering the expression or structure of an individual's genetic makeup. On the other hand, nutrigenetics is significantly different from nutrigenomics since nutrigenetics has been used for decades in certain rare monogenic diseases such as phenylketonuria, and has the potential to provide a basis for personalized dietary recommendation based on the individual's specific genetic background in order to prevent common multifactorial disorders decades before their clinical manifestation. The human genome maps and SNP databases, together with the rapid development of tools suitable for investigating genetic and epigenetic changes in small tissue biopsies provide the means to begin the test hypothesis about the mechanisms by which diet influences disease risk including cancer directly in human subjects, could be inevitable flatforms for clinical application to achieve targeted therapy in near future.
Keyword
MeSH Terms
Figure
Reference
-
1. Stover PJ. Nutritional genomics. Physiol Genomics. 2004. 16:161–165.
Article2. Davis CD, Milner J. Frontiers in nutrigenomics, proteomics, metabolomics and cancer prevention. Mutat Res. 2004. 551:51–64.
Article3. Kaput J, Rodriguez RL. Nutritional genomics: the next frontier in the postgenomic era. Physiol Genomics. 2004. 16:166–177.
Article4. Elliott R, Ong TJ. Nutritional genomics. BMJ. 2002. 324:1438–1442.5. Muller M, Kersten S. Nutrigenomics: goals and strategies. Nat Rev Genet. 2003. 4:315–322.
Article6. Liu-Stratton Y, Roy S, Sen CK. DNA microarray technology in nutraceutical and food safety. Toxicol Lett. 2004. 150:29–42.
Article7. Fenech M. The role of folic acid and Vitamin B12 in genomic stability of human cells. Mutat Res. 2001. 475:57–67.
Article8. Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001. 475:69–87.
Article9. Halliwell B. Vitamin C and genomic stability. Mutat Res. 2001. 475:29–35.
Article10. Claycombe KJ, Meydani SN. Vitamin E and genome stability. Mutat Res. 2001. 475:37–44.
Article11. Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA. 1991. 88:11003–11006.
Article12. Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rozen R, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996. 93:7–9.
Article13. Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000. 72:324–332.
Article14. Ordovas JM, Mooser V. Nutrigenomics and nutrigenetics. Curr Opin Lipidol. 2004. 15:101–108.
Article15. Mathers JC. The biological revolution-towards a mechanistic understanding of the impact of diet on cancer risk. Mutat Res. 2004. 55:43–49.16. IHGSC. Initial sequencing and analysis of the human genome. Nature. 2001. 409:860–921.17. Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Holt RA, et al. The sequence of the human genome. Science. 2001. 291:1304–1351.
Article18. Roy S, Khanna S, Bentley K, Beffrey P, Sen CK. Functional genomics: high-density oligonucleotide arrays. Methods Enzymol. 2002. 353:487–497.
Article19. Roy S, Lado BH, Khanna S, Sen CK. Vitamin E sensitive genes in the developing rat fetal brain: a high-density oligonucleotide microarray analysis. FEBS Lett. 2002. 530:17–23.
Article20. Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004. 3:19.
Article21. Fairweather-Tait SJ. Human nutrition and food research: opportunities and challenges in the post-genomic era. Philos Trans R Soc Lond B Biol Sci. 2003. 358:1709–1727.
Article22. Kaput J. Diet-disease gene interactions. Nutrition. 2004. 20:26–31.
Article23. Kornman KS, Martha PM, Duff GW. Genetic variations and inflammation: a practical nutrigenomics opportunity. Nutrition. 2004. 20:44–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Nutrigenomics in Diabetes
- Nutritional Approach for Integrative Medicine
- The Effect of Caffeine on 3T3-L1 Adipocyte Differentiation : A Nutrigenomical Approach
- Genome-Wide Association Studies of the Korea Association REsource (KARE) Consortium
- A Comparative Study between the Application Group and Non-application Group of a Sand Bag on the Surgical Region after a Pediatric Cardiac Catheterization