J Korean Med Assoc.
2006 Jan;49(1):30-40. 10.5124/jkma.2006.49.1.30.
Pharmacological Therapy for Postmenopausal Osteoporosis
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine and Hospital, Korea. csshin@snu.ac.kr
- KMID: 2184633
- DOI: http://doi.org/10.5124/jkma.2006.49.1.30
Abstract
- Osteoporosis, now defined as a disease characterized by a low bone mass and a microarchitectural deterioration of bone tissue leading to an enhanced bone fragility and fracture risk, is a major public health problem, affecting 200 million individuals worldwide. Optimal treatment and prevention of osteoporosis require modification of risk factors, particularly smoking cessation, adequate physical activity, and attention to diet, in addition to pharmacologic intervention. A number of pharmacologic options are now available to health care providers. The estrogens and raloxifene both prevent bone loss in postmenopausal women, and the estrogens probably also decrease the risk of first fracture. There is a good evidence that raloxifene prevents further fractures in postmenopausal women who already have had fractures and some evidence that estrogen does as well. Bisphosphonate alendronate prevents bone loss and reduces fractures in healthy and osteoporotic postmenopausal women, and in osteoporotic men as well. Risedronate is more potent and has fewer upper gastrointestinal side effects than alendronate, and reduces the incidence of fractures in osteoporotic women. An intermittent use of potent bisphosphonate zoledronate also increases bone mineral density and may become an alternative in the prevention and treatment of osteoporosis. Calcitonin increases bone mineral density in early postmenopausal women and men with idiopathic osteoporosis, and also reduces the risk of new fractures in osteoporotic women. All of the agents discussed above prevent bone resorption, whereas teriparatide increases bone formation and is effective in the treatment of osteoporotic women and men. Bisphosphonates are also effective in the treatment of secondary osteoporosis associated with the use of glucocorticoids to treat inflammation or prevent rejection after transplantation. New avenues for targeting osteoporosis will emerge as our knowledge of the regulatory mechanisms of bone remodelling increases, although issues of tissue specificity may remain to be solved.
MeSH Terms
-
Alendronate
Bone and Bones
Bone Density
Bone Resorption
Calcitonin
Diet
Diphosphonates
Estrogens
Female
Glucocorticoids
Health Personnel
Humans
Incidence
Inflammation
Male
Motor Activity
Organ Specificity
Osteogenesis
Osteoporosis
Osteoporosis, Postmenopausal*
Public Health
Raloxifene Hydrochloride
Risedronate Sodium
Risk Factors
Smoking Cessation
Teriparatide
Alendronate
Calcitonin
Diphosphonates
Estrogens
Glucocorticoids
Teriparatide
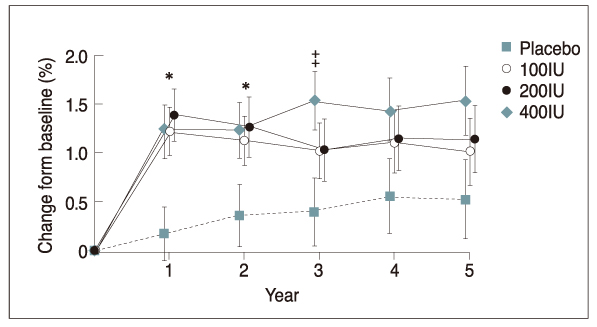
Figure
Reference
-
1. Recker RR, Hinders S, Davies KM, Heaney RP, Stegman MR, Kimmel DB, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996. 11:1961–1966.
Article2. Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: a randomized controlled trial [see comments]. Am J Med. 1995. 98:331–335.
Article3. Nieves JW, Komar L, Cosman F, Lindsay R. Calcium potentiates the effect of estrogen and calcitonin on bone mass: review and analysis [see comments]. Am J Clin Nutr. 1998. 67:18–24.
Article4. Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997. 12:935–941.
Article5. Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980. 51:1359–1364.
Article6. The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995. 274(21):199–208. [see comments] [published erratum appears in JAMA 1995 Dec 6; 274(21): 1676].7. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, Watts NB, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003. 290:1729–1738.
Article8. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Wassertheil-Smoller S, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004. 291:1701–1712.
Article9. Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003. 289:2673–2684.
Article10. Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Baylink D, et al. PROOF Study Group. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. Am J Med. 2000. 109:267–276.
Article11. Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Yanover MJ, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis [see comments]. N Engl J Med. 1990. 323:73–79.
Article12. Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Favus M, et al. The Alendronate Phase III Osteoporosis Treatment Study Group. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group [see comments]. N Engl J Med. 1995. 333:1437–1443. [see comments].
Article13. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Ensrud KE, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996. 348:1535–1541. [see comments].
Article14. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Miller PD, et al. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999. 282:1344–1352.
Article15. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Cummings SR, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999. 282:637–645. [see comments].
Article16. Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989. 83:60–65.
Article17. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Mitlak BH, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001. 344:1434–1441.
Article18. Marie PJ. Optimizing bone metabolism in osteoporosis: insight into the pharmacologic profile of strontium ranelate. Osteoporos Int. 2003. 14:Suppl 3. S9–S12.
Article19. Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Reginster JY, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004. 350:459–468.
Article