J Breast Cancer.
2014 Dec;17(4):363-369. 10.4048/jbc.2014.17.4.363.
Risk of Trastuzumab-Related Cardiotoxicity in Early Breast Cancer Patients: A Prospective Observational Study
- Affiliations
-
- 1Department of Cardiology, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China. xiaozhongzhang06@sina.com
- 2Department of Breast Cancer, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China.
- KMID: 2176128
- DOI: http://doi.org/10.4048/jbc.2014.17.4.363
Abstract
- PURPOSE
In the present study, we investigated the incidence of cardiotoxicity within 5 years of trastuzumab treatment and evaluated potential risk factors in clinical practice.
METHODS
The study cohort included 415 patients diagnosed with early breast cancer (EBC). Cardiotoxicity incidence was evaluated in patients receiving trastuzumab and those who did not. Multivariate Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals of potential risk factors for trastuzumab-related cardiotoxicity after appropriate adjustments.
RESULTS
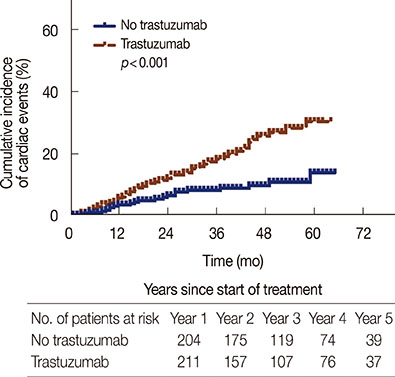
Incidence of cardiotoxicity in patients treated with trastuzumab was significantly higher than that in controls (23.7% vs. 10.8%, p<0.001). This result was adjusted for factors that might increase the risk of cardiotoxicity, such as history of coronary artery diseases or the use of anthracyclines for more than four cycles.
CONCLUSION
Our findings indicated that treatment with trastuzumab was strongly associated with cardiotoxicity in EBC patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cardioprotective Effect of Dexrazoxane in Patients with HER2-Positive Breast Cancer Who Receive Anthracycline Based Adjuvant Chemotherapy Followed by Trastuzumab
In-Ho Kim, Ji Eun Lee, Ho-Joong Youn, Byung Joo Song, Byung Joo Chae
J Breast Cancer. 2017;20(1):82-90. doi: 10.4048/jbc.2017.20.1.82.
Reference
-
1. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005; 353:1659–1672.2. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–792.
Article3. Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol. 2012; 23:3058–3063.
Article4. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002; 20:1215–1221.
Article5. Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008; 26:1231–1238.
Article6. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012; 30:3792–3799.7. Huszno J, Leś D, Sarzyczny-Słota D, Nowara E. Cardiac side effects of trastuzumab in breast cancer patients: single centere experiences. Contemp Oncol Pozn. 2013; 17:190–195.8. Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013; 31:4222–4228.
Article9. Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart. 2013; 99:634–639.
Article10. Martín M, Esteva FJ, Alba E, Khandheria B, Pérez-Isla L, García-Sáenz JA, et al. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009; 14:1–11.
Article11. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365:1273–1283.
Article12. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012; 104:1293–1305.
Article13. Zambelli A, Della Porta MG, Eleuteri E, De Giuli L, Catalano O, Tondini C, et al. Predicting and preventing cardiotoxicity in the era of breast cancer targeted therapies: novel molecular tools for clinical issues. Breast. 2011; 20:176–183.
Article14. Du XL, Xia R, Burau K, Liu CC. Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998-2005. Med Oncol. 2011; 28:Suppl 1. S80–S90.
Article15. Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005; 23:7811–7819.
Article16. Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol. 2013; 20:443–464.
Article17. Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. 2009; 27:2638–2644.
Article18. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013; 368:987–998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trastuzumab Exposure during Pregnancy with Invasive Breast Cancer
- Early Cardiac Function Monitoring for Detection of Subclinical Doxorubicin Cardiotoxicity in Young Adult Patients with Breast Cancer
- MicroRNA-130a Increases and Predicts Cardiotoxicity during Adjuvant Chemotherapy in Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer
- Clinical Exercise Prescription for Cardiovascular Health in Breast Cancer Survivors
- Chemotherapy-Induced Left Ventricular Dysfunction in Patients with Breast Cancer