J Breast Cancer.
2011 Mar;14(1):39-45. 10.4048/jbc.2011.14.1.39.
Prognostic Factors in Patients with Stage II/III Breast Cancer Treated with Adjuvant Extension of Neoadjuvant Chemotherapy: A Retrospective Cohort Study with Ten-Years of Follow-Up Data
- Affiliations
-
- 1Department of Surgery, Research Institute for Medical Science, Chungnam National University College of Medicine, Daejeon, Korea. kimjr@cnu.ac.kr
- 2Department of Pathology, Research Institute for Medical Science, Chungnam National University College of Medicine, Daejeon, Korea.
- KMID: 2175641
- DOI: http://doi.org/10.4048/jbc.2011.14.1.39
Abstract
- PURPOSE
The aim of this retrospective study was to identify the reliable long term prognostic factors in patients with stage II/III breast cancer who were treated with an adjuvant extension of neoadjuvant chemotherapy (NC).
METHODS
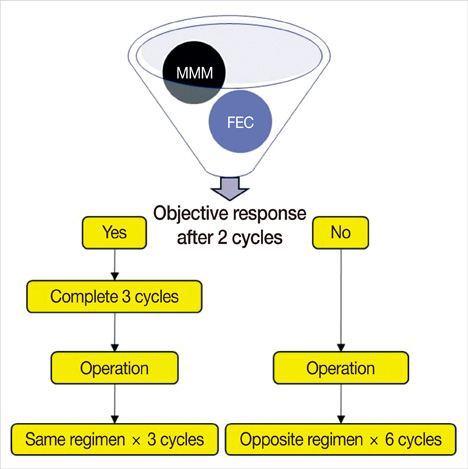
Women under the age of 70-years, with previously untreated clinical stage II and III breast cancer, were treated with NC, which was comprised of three cycles of FEC (5-FU, epirubicin, and cyclophosphamide every 3 weeks) or MMM (methotrexate, mitoxantrone, and mitomycin-C every 3 weeks) with an adjuvant extension of three cycles of the same regimen.
RESULTS
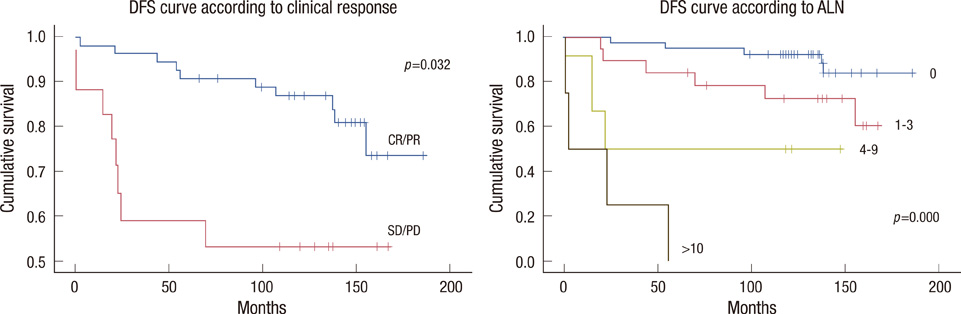
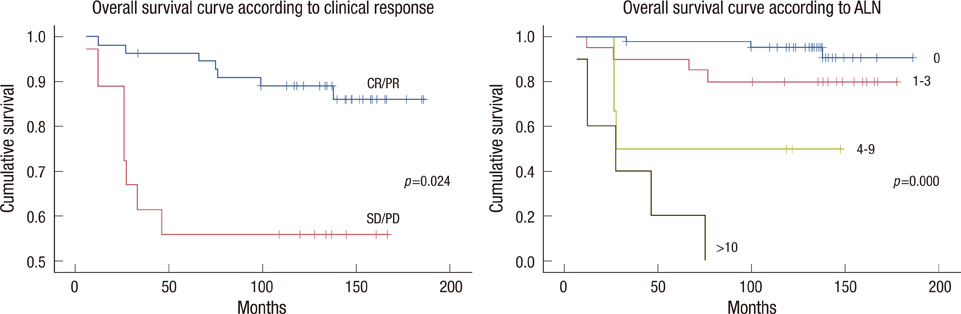
Cumulative 10-years disease-free survival (DFS) was 87.3% for patients with a good response and 55.5% for patients with no response (p=0.032); 92.9% for node negative patients, 75.0% for 1-3 positive nodes, 50.0% for 4-9 positive nodes and no survival for 10 or more positive nodes (p<0.001). Cumulative 10-years overall survival (OS) was 89.1% for patients with good response and 55.5% for patients with no response (p=0.024); 95.2% for node negative patients, 80.0% for 1-3 positive nodes, 50.0% for 4-9 positive nodes and no survival for 10 or more positive nodes (p<0.001). No significant difference was observed in DFS and OS between the FEC and MMM treated groups.
CONCLUSION
Based on a review of data with a long follow-up, only the clinical response to NC and the absolute number of metastatic axillary lymph node identified at surgical staging were independent predictors of both DFS and OS in patients with stage II/III breast cancer patients treated with adjuvant extension of NC.
Keyword
MeSH Terms
Figure
Reference
-
1. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997. 15:2483–2493.
Article2. Hortobagyi GN, Blumenschein GR, Spanos W, Montague ED, Buzdar AU, Yap HY, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983. 51:763–768.
Article3. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999. 17:460–469.
Article4. Mamounas EP. NSABP Protocol B-27. Preoperative doxorubicin plus cyclophosphamide followed by preoperative or postoperative docetaxel. Oncology (Williston Park). 1997. 11:6 Suppl 6. 37–40.5. Kimura M, Sano M, Fujimori M, Nakagomi H, Negishi T, Yanagita Y, et al. Neoadjuvant paclitaxel for operable breast cancer: multicenter phase II trial with clinical outcomes. Anticancer Res. 2008. 28:1239–1244.6. Kaufmann P, Dauphine CE, Vargas MP, Burla ML, Isaac NM, Gonzalez KD, et al. Success of neoadjuvant chemotherapy in conversion of mastectomy to breast conservation surgery. Am Surg. 2006. 72:935–938.
Article7. Forrest AP, Levack PA, Chetty U, Hawkins RA, Miller WR, Smyth JF, et al. A human tumour model. Lancet. 1986. 2:840–842.
Article8. Kim JR, Chang ES. Prognostic factors in breast cancer patients following neoadjuvant chemotherapy. J Korean Surg Soc. 2000. 59:729–737.
Article9. The Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2002. J Korean Breast Cancer Soc. 2004. 7:72–83.10. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007. 7:203.
Article11. Jacquillat C, Weil M, Baillet F, Borel C, Auclerc G, de Maublanc MA, et al. Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer. Cancer. 1990. 66:119–129.
Article12. Fernandez-Sanchez M, Gamboa-Dominguez A, Uribe N, Garcia-Ulloa AC, Flores-Estrada D, Candelaria M, et al. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol. 2006. 23:171–183.
Article13. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008. 26:778–785.
Article14. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001. 19:4224–4237.
Article15. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005. 97:188–194.
Article16. Knauer M, Haid A, Schneider Y, Köberle-Wührer R, Lang A, Winder T, et al. Adjuvant extension of chemotherapy after neoadjuvant therapy may not improve outcome in early-stage breast cancer. Eur J Surg Oncol. 2009. 35:798–804.
Article17. Han S, Kim J, Lee J, Chang E, Gwak G, Cho H, et al. Comparison of 6 cycles versus 4 cycles of neoadjuvant epirubicin plus docetaxel chemotherapy in stages II and III breast cancer. Eur J Surg Oncol. 2009. 35:583–587.
Article18. Steger GG, Galid A, Gnant M, Mlineritsch B, Lang A, Tausch C, et al. Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG-14. J Clin Oncol. 2007. 25:2012–2018.
Article19. Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010. 28:2024–2031.
Article20. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010. 375:377–384.
Article21. Mathew J, Asgeirsson KS, Agrawal A, Mukherjee A, Ellis IO, Cheung KL, et al. Neoadjuvant chemotherapy in locally advanced primary breast cancers: the Nottingham experience. Eur J Surg Oncol. 2007. 33:972–976.
Article22. Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008. 26:814–819.
Article23. Singletary SE. Neoadjuvant chemotherapy in the treatment of stage II and III breast cancer. Am J Surg. 2001. 182:341–346.
Article24. Daveau C, Stevens D, Brain E, Berges O, Villette S, Moisson P, et al. Is regional lymph node irradiation necessary in stage II to III breast cancer patients with negative pathologic node status after neoadjuvant chemotherapy? Int J Radiat Oncol Biol Phys. 2010. 78:337–342.
Article25. Schwartz GF, Tannebaum JE, Jernigan AM, Palazzo JP. Axillary sentinel lymph node biopsy after neoadjuvant chemotherapy for carcinoma of the breast. Cancer. 2010. 116:1243–1251.
Article26. Pierga JY, Mouret E, Laurence V, Dieras V, Savigioni A, Beuzeboc P, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. the role of clinical response. Eur J Cancer. 2003. 39:1089–1096.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Using the Lymph Node Ratio to Evaluate the Prognosis of Stage II/III Breast Cancer Patients Who Received Neoadjuvant Chemotherapy and Mastectomy
- Neoadjuvant Chemotherapy for the Local Advanced Breast Cancer
- Adjuvant therapy in cervical cancer patients with high risk factors
- Osteosarcoma, survivorship following stage and chemotherapeutic regimen: 13 year experience of Korea Cancer Center Hospital
- Prognostic Factor and Survival Benefit of Adjuvant Chemotherapy in Stage IIA Colon Cancer