Intest Res.
2014 Jul;12(3):229-235. 10.5217/ir.2014.12.3.229.
Prediction of Tumor Recurrence in Patients with Non-Gastric Gastrointestinal Stromal Tumors Following Resection according to the Modified National Institutes of Health Criteria
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea. jp-im@hanmail.net
- KMID: 2174382
- DOI: http://doi.org/10.5217/ir.2014.12.3.229
Abstract
- BACKGROUND/AIMS
Few studies have investigated the prognosis of non-gastric gastrointestinal stromal tumors (GISTs) under the modified National Institutes of Health (NIH) consensus criteria in Korea. This study aims to clarify the clinical usefulness of the modified NIH criteria for risk stratification.
METHODS
From January 2000 through October 2012, 88 patients who underwent curative resection for primary GISTs were included in this study. The enrolled patients were stratified to predict recurrence by the original NIH criteria and modified NIH criteria.
RESULTS
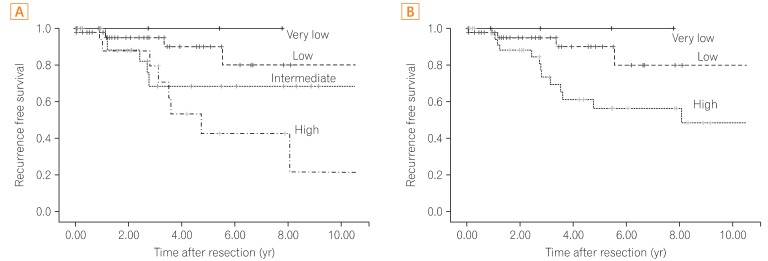
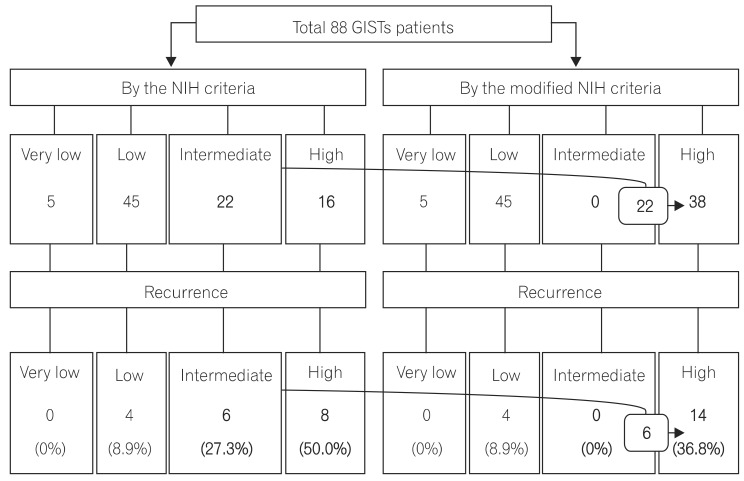
In all, 88 patients had non-gastric GISTs, including 82 and 6 patients with GISTs of the small intestine and colorectum, respectively. The mean age was 57.3+/-13.0 years, and the median follow-up duration was 3.40 years (range, 0.02-12.76 years). All patients who were placed in the intermediate-risk category according to the original NIH criteria were reclassified into the high-risk category according to the modified NIH criteria. Therefore, the proportion of cases in the intermediate-risk category declined to 0.0% from 25.0% (22/88), and the proportion of cases in the high-risk category increased to 43.2% (38/88) from 18.2% (16/88) under the modified NIH criteria. Among the 22 reclassified patients, 6 (27.3%) suffered a recurrence during the observational period, and the recurrence rate of high-risk category patients was 36.8% (14/38).
CONCLUSIONS
Patients in the high-risk category according to the modified NIH criteria had a high GIST recurrence rate. Therefore, the modified NIH criteria are clinically useful in selecting patients who need imatinib adjuvant chemotherapy after curative surgical resection.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A High Risk Group in the Modified National Institutes of Health Consensus Criteria for the Gastrointestinal Stromal Tumor: A Clear Indication of the Adjuvant Imatinib
Dong Kyung Chang
Intest Res. 2014;12(3):176-177. doi: 10.5217/ir.2014.12.3.176.
Reference
-
1. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130:1466–1478. PMID: 17090188.
Article2. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998; 279:577–580. PMID: 9438854.
Article3. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465. PMID: 12094370.
Article4. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29:52–68. PMID: 15613856.5. Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006; 30:477–489. PMID: 16625094.6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83. PMID: 17193820.
Article7. Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007; 14:2018–2027. PMID: 17473953.
Article8. Joensuu H. Predicting recurrence-free survival after surgery for GIST. Lancet Oncol. 2009; 10:1025. PMID: 19793677.
Article9. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419. PMID: 18774375.
Article10. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8(Suppl 2):S1–S41. PMID: 20457867.
Article11. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480. PMID: 12181401.
Article12. von Mehren M, Benjamin RS, Bui MM, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012; 10:951–960. PMID: 22878820.13. Reynoso D, Trent JC. Neoadjuvant and adjuvant imatinib treatment in gastrointestinal stromal tumor: current status and recent developments. Curr Opin Oncol. 2010; 22:330–335. PMID: 20520542.
Article14. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009; 373:1097–1104. PMID: 19303137.
Article15. Hohenberger P. Adjuvant imatinib in GIST: a self-fulfilling prophecy, or more? Lancet. 2009; 373:1058–1060. PMID: 19303138.
Article16. Gronchi A, Judson I, Nishida T, et al. Adjuvant treatment of GIST with imatinib: solid ground or still quicksand? A comment on behalf of the EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group, the NCRI Sarcoma Clinical Studies Group (UK), the Japanese Study Group on GIST, the French Sarcoma Group and the Spanish Sarcoma Group (GEIS). Eur J Cancer. 2009; 45:1103–1106. PMID: 19286368.
Article17. Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012; 13:265–274. PMID: 22153892.
Article18. Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011; 37:890–896. PMID: 21737227.
Article19. Joensuu H. Adjuvant therapy for high-risk gastrointestinal stromal tumour: considerations for optimal management. Drugs. 2012; 72:1953–1963. PMID: 22994537.
Article20. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009; 99:42–47. PMID: 18942073.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A High Risk Group in the Modified National Institutes of Health Consensus Criteria for the Gastrointestinal Stromal Tumor: A Clear Indication of the Adjuvant Imatinib
- Interpretation of Pathologic Margin after Endoscopic Resection of Gastrointestinal Stromal Tumor
- Long-Term Outcomes after Endoscopic Treatment of Gastric Gastrointestinal Stromal Tumor
- Surgical Treatment of Gastric Gastrointestinal Stromal Tumor
- Tumor Size is Associated with Long-term Outcomes after Resection of Gastric Gastrointestinal Stromal Tumors