J Gynecol Oncol.
2009 Sep;20(3):151-157. 10.3802/jgo.2009.20.3.151.
Acute toxicity of cyclooxygenase-2 inhibitor rofecoxib as a radiosensitizer for concurrent chemoradiation in the treatment of uterine cervical cancer
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Women's Cancer Clinic, Yonsei University College of Medicine, Seoul, Korea. ytkchoi@yuhs.ac
- 2Department of Obstetrics and Gynecology, Kwandong University College of Medicine, Myongji Hospital, Goyang, Korea.
- KMID: 2173447
- DOI: http://doi.org/10.3802/jgo.2009.20.3.151
Abstract
OBJECTIVE
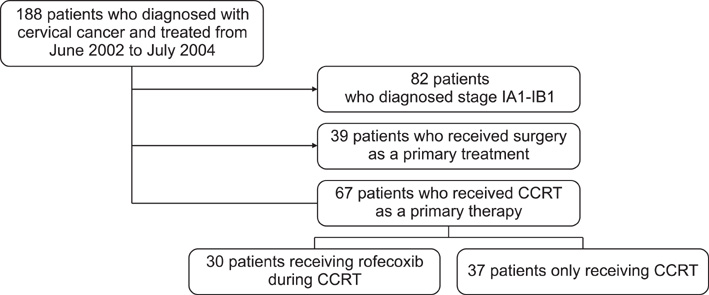
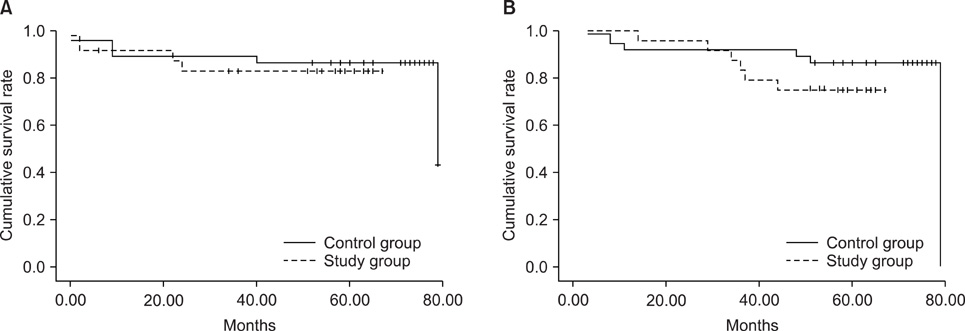
To evaluate the acute toxicity of rofecoxib during concurrent use with cisplatin-based chemoradiotherapy (CCRT) in patients with cervical cancer. METHODS: We evaluated 67 FIGO stage IB2-IVA cervical cancer patients treated with CCRT between June 2002 and July 2004. The study group included patients who received rofecoxib (N=30) and the control group included patients who received CCRT only (N=37). The patients' medical records were retrospectively reviewed for patient characteristics, toxicity related to CCRT and treatment results. RESULTS: There were no significant differences in toxicity between the two groups. The most common acute grade 3/4 toxicity was neutropenia (13.3% in the study group and 21.6% in the control group). Grade 3/4 late toxicity was observed in 2 (6.6%) patients in the study group and 3 (8.1%) in the control group. There was no treatment-related deaths in either group. Six (20.0%) patients in the study group had treatment failure. In the control group, 6 (16.2%) patients experienced treatment failure. Progression-free and overall survival was 55.8+/-4.2 and 59.0+/-2.8 months, respectively, in the study group, and 69.7+/-4.3 and 71.6+/-3.6 months, respectively, in the control group. There were no differences in progression-free and overall survival between the 2 groups. CONCLUSION: Our data indicate that rofecoxib, at a dose of 25 mg twice daily, has acceptable acute toxicity as a radiosensitizer during CCRT. Although rofecoxib was not efficacious as a radiosensitizer in the present study, the benefit of rofecoxib as a radiosensitizer should be further evaluated in a prospective study.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009. 20:1–7.2. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999. 340:1154–1161.3. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999. 340:1137–1143.4. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999. 340:1144–1153.5. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999. 17:1339–1348.6. Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000. 18:1606–1613.7. Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007. 26:525–534.8. Lee JS, Choi YD, Lee JH, Nam JH, Choi C, Lee MC, et al. Expression of cyclooxygenase-2 in adenocarcinomas of the uterine cervix and its relation to angiogenesis and tumor growth. Gynecol Oncol. 2004. 95:523–529.9. Ishiko O, Sumi T, Yoshida H, Matsumoto Y, Honda K, Deguchi M, et al. Association between overexpression of cyclooxygenase-2 and suppression of apoptosis in advanced cancer of the uterine cervix after cyclic balloon-occluded arterial infusion. Oncol Rep. 2001. 8:1259–1263.10. Fujimoto J, Toyoki H, Sato E, Sakaguchi H, Jahan I, Alam SM, et al. Expression of cyclooxygenase-2 related to angiogenesis in uterine cervical cancers. J Biomed Sci. 2006. 13:825–832.11. Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC, Oh KS. High cyclooxygenase-2 expression in stage IB cervical cancer with lymph node metastasis or parametrial invasion. Gynecol Oncol. 2000. 76:320–325.12. Lindstrom AK, Stendahl U, Tot T, Lidström BM, Hellberg D. Predicting the outcome of squamous cell carcinoma of the uterine cervix using combinations of individual tumor marker expressions. Anticancer Res. 2007. 27:1609–1615.13. Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR, Lee JD, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res. 2004. 10:1366–1374.14. Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ, Chang SK, et al. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002. 95:531–539.15. Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002. 20:973–981.16. Kim YS, Shin SS, Nam JH, Kim YT, Kim YM, Kim JH, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008. 108:195–200.17. Gaffney DK, Winter K, Dicker AP, Miller B, Eifel PJ, Ryu J, et al. A Phase II study of acute toxicity for Celebrex (celecoxib) and chemoradiation in patients with locally advanced cervical cancer: primary endpoint analysis of RTOG 0128. Int J Radiat Oncol Biol Phys. 2007. 67:104–109.18. Herrera FG, Chan P, Doll C, Milosevic M, Oza A, Syed A, et al. A prospective phase I-II trial of the cyclooxygenase-2 inhibitor celecoxib in patients with carcinoma of the cervix with biomarker assessment of the tumor microenvironment. Int J Radiat Oncol Biol Phys. 2007. 67:97–103.19. Hefler LA, Grimm C, Speiser P, Sliutz G, Reinthaller A. The cyclooxygenase-2 inhibitor rofecoxib (Vioxx) in the treatment of cervical dysplasia grade II-III A phase II trial. Eur J Obstet Gynecol Reprod Biol. 2006. 125:251–254.20. Gaffney DK, Winter K, Dicker AP, Miller B, Eifel PJ, Ryu J, et al. Efficacy and patterns of failure for locally advanced cancer of the cervix treated with celebrex (celecoxib) and chemoradiotherapy in RTOG 0128. Int J Radiat Oncol Biol Phys. 2007. 69:111–117.21. Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000. 342:1946–1952.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of primary retroperitoneal undifferentiated endometrial stromal sarcoma after concurrent chemoradiation therapy for cervical cancer
- Treatment of Cervical Cancer
- Variable uterine uptake of FDG in adenomyosis during concurrent chemoradiation therapy for cervical cancer
- Comparison of Concurrent Chemoradiotherapy Regimen Toxicities in the Treatment of Loco-Regionally Advanced Cervical Cancer
- Concurrent Chemoradiotherapy in Cervical Cancer (A New Paradigm in Cervical Cancer Treatment)