Blood Res.
2013 Dec;48(4):266-273. 10.5045/br.2013.48.4.266.
Prognostic value of immunohistochemical algorithms in gastrointestinal diffuse large B-cell lymphoma
- Affiliations
-
- 1Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. jrhuh@amc.seoul.kr
- 2Department of Oncology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 2172874
- DOI: http://doi.org/10.5045/br.2013.48.4.266
Abstract
- BACKGROUND
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous clinicopathological entity, and its molecular classification into germinal center B cell-like (GCB) and activated B cell-like (ABC) subtypes using gene expression profile analysis has been shown to have prognostic significance. Recent attempts have been made to find an association between immunohistochemical findings and molecular subgroup, although the clinical utility of immunohistochemical classification remains uncertain.
METHODS
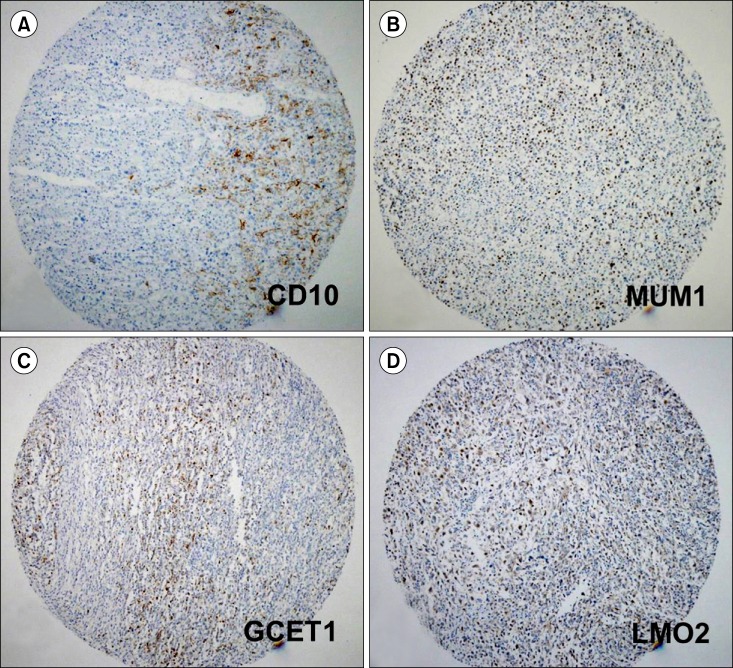
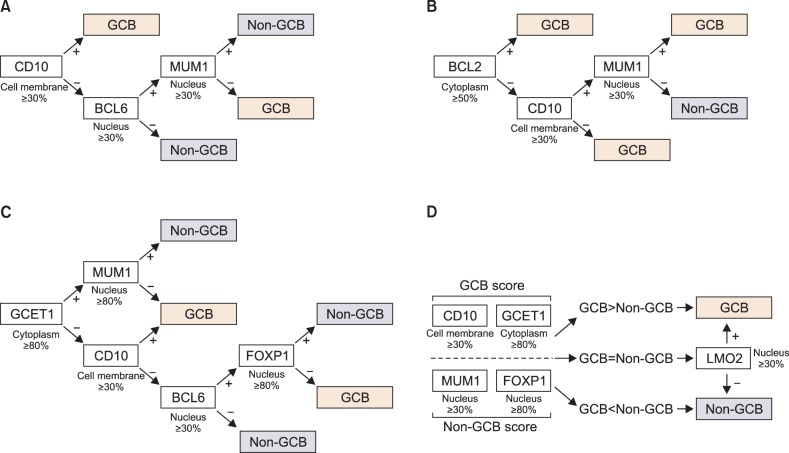
The clinicopathological features and follow-up data of 68 cases of surgically resected gastrointestinal DLBCL were analyzed. Using the immunohistochemical findings on tissue microarray, the cases were categorized into GCB and non-GCB subtypes according to the algorithms proposed by Hans, Muris, Choi, and Tally.
RESULTS
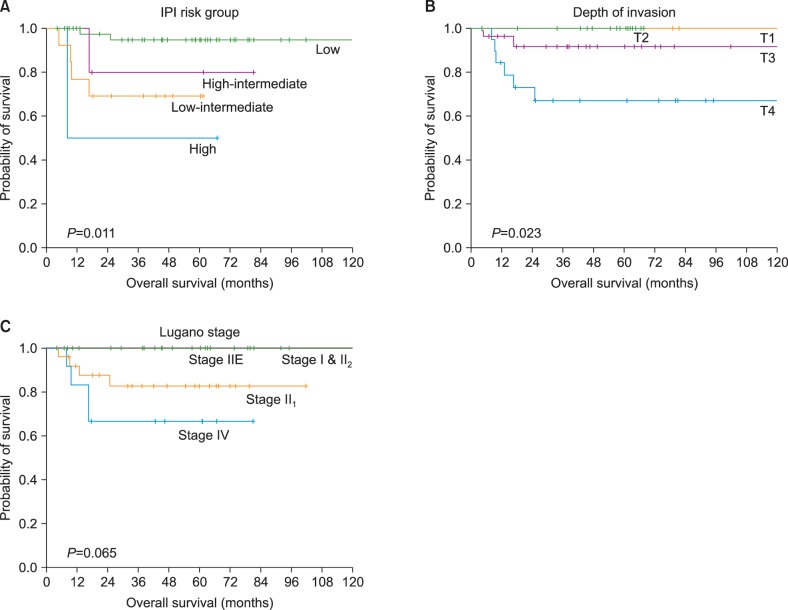
The median patient age was 56 years (range, 26-77 years). Of the 68 cases included, 39.7% (27/68) involved the stomach, and 60.3% (41/68) involved the intestines. The GCB and non-GCB groups sorted according to Hans, Choi, and Tally algorithms, but not the Muris algorithm, were closely concordant (Hans vs. Choi, kappa=0.775, P<0.001; Hans vs. Tally, kappa=0.724, P<0.001; Choi vs. Tally, kappa=0.528, P<0.001). However, there was no prognostic difference between the GCB and non-GCB subtypes, regardless of the algorithm used. On univariate survival analyses, international prognostic index risk group and depth of tumor invasion both had prognostic significance.
CONCLUSION
The Hans, Choi, and Tally algorithms might represent identical DLBCL subgroups, but this grouping did not correlate with prognosis. Further studies may delineate the association between immunohistochemical subgroups and prognosis.
MeSH Terms
Figure
Reference
-
1. Stein H, Warnke RA, Chan WC, et al. Diffuse large B-cell lymphoma, not otherwise specified. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC;2008. p. 233–237.2. Armitage JO, Fyfe MA, Lewis J. Long-term remission durability and functional status of patients treated for diffuse histiocytic lymphoma with the CHOP regimen. J Clin Oncol. 1984; 2:898–902. PMID: 6379123.
Article3. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1002–1006. PMID: 7680764.
Article4. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005; 23:4117–4126. PMID: 15867204.
Article5. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–391. PMID: 16648042.6. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329:987–994. PMID: 8141877.7. Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006; 24:995–1007. PMID: 16418498.
Article8. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403:503–511. PMID: 10676951.
Article9. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:1937–1947. PMID: 12075054.10. Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008; 359:2313–2323. PMID: 19038878.
Article11. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–282. PMID: 14504078.
Article12. Muris JJ, Meijer CJ, Vos W, et al. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006; 208:714–723. PMID: 16400625.
Article13. Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009; 15:5494–5502. PMID: 19706817.
Article14. Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppa S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009; 22:1094–1101. PMID: 19448593.
Article15. Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011; 29:200–207. PMID: 21135273.
Article16. Dupuis J, Gaulard P, Hemery F, et al. Respective prognostic values of germinal center phenotype and early (18)fluorodeoxyglucose-positron emission tomography scanning in previously untreated patients with diffuse large B-cell lymphoma. Haematologica. 2007; 92:778–783. PMID: 17550850.
Article17. Veelken H, Vik Dannheim S, Schulte Moenting J, Martens UM, Finke J, Schmitt-Graeff A. Immunophenotype as prognostic factor for diffuse large B-cell lymphoma in patients undergoing clinical risk-adapted therapy. Ann Oncol. 2007; 18:931–939. PMID: 17395602.
Article18. Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011; 117:4836–4843. PMID: 21441466.19. Rayman N, Lam KH, van der Holt B, et al. Prognostic relevance of immunohistochemical subclassification of diffuse large B-cell lymphoma in two prospective phase III clinical trials. Clin Lymphoma Myeloma Leuk. 2011; 11:23–32. PMID: 21454187.
Article20. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging handbook. 7th ed. New York, NY: Springer;2010.21. Cheson BD. New response criteria for lymphomas in clinical trials. Ann Oncol. 2008; 19(Suppl 4):iv35–iv38. PMID: 18519399.
Article22. Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009; 113:6069–6076. PMID: 19380866.
Article23. Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011; 29:690–697. PMID: 21189393.
Article24. Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004; 350:1828–1837. PMID: 15115829.
Article25. Chen YW, Hu XT, Liang AC, et al. High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood. 2006; 108:2373–2383. PMID: 16772602.
Article26. Iqbal J, Neppalli VT, Wright G, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006; 24:961–968. PMID: 16418494.27. Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006; 107:4207–4213. PMID: 16449523.
Article28. Natkunam Y, Zhao S, Mason DY, et al. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007; 109:1636–1642. PMID: 17038524.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Advanced Gastric Adenocarcinoma with Synchronous Gastric Diffuse Large B Cell Lymphoma
- A Case of Primary Rectal Diffuse Large B Cell LymphomaPresented as Multiple Polypoid Lesions
- A Case of Secondary Cutaneous Diffuse Large B-cell Lymphoma
- A Case of Primary Cutaneous Diffuse Large B-cell Lymphoma
- Relapse of Ocular Lymphoma following Primary Testicular Diffuse Large B-cell Lymphoma