Ann Dermatol.
2011 Feb;23(1):33-38. 10.5021/ad.2011.23.1.33.
Expression of the c-Met Proteins in Malignant Skin Cancers
- Affiliations
-
- 1Molecular Cancer Research Center, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 2Department of Dermatology, Soonchunhyang University Hospital, Seoul, Korea. mkcho2001@hanmail.net
- KMID: 2171949
- DOI: http://doi.org/10.5021/ad.2011.23.1.33
Abstract
- BACKGROUND
The expression of c-Met is substantially elevated in most malignant human cancers. We therefore searched for c-Met expression and compared the expression level among malignant skin cancers.
OBJECTIVE
The aim of this study was to determine the c-Met expression pattern and the protein expression level in selected malignant cutaneous tumors.
METHODS
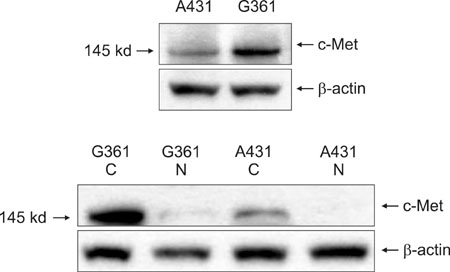
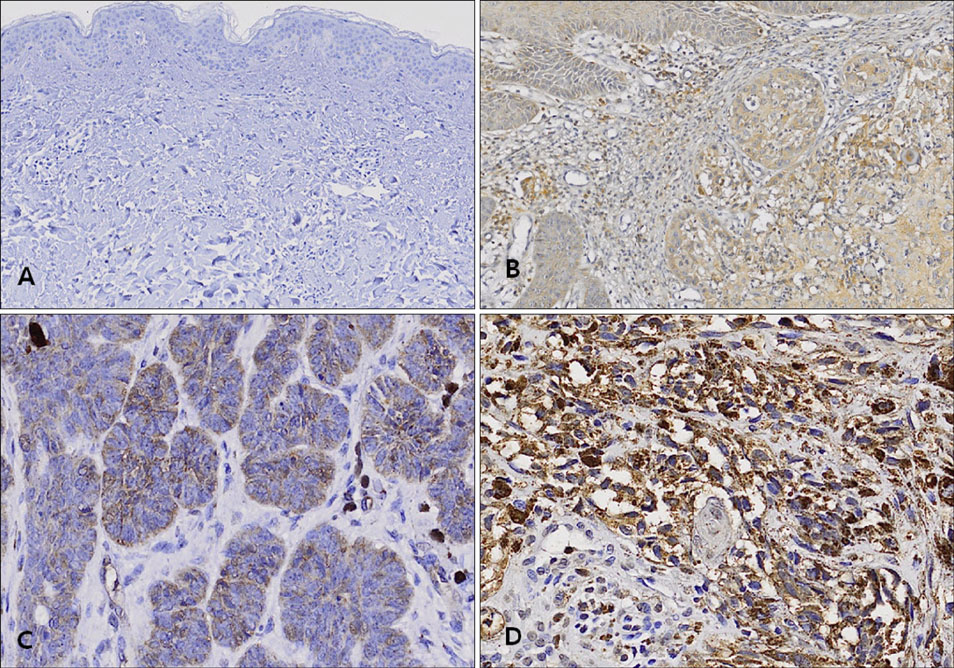
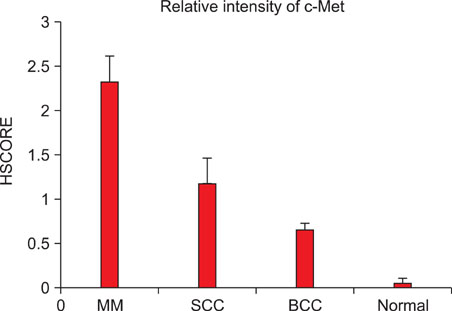
G361 cells (malignant melanoma cell line) and A431 cells (squamous cell carcinoma cell line) were cultured and analyzed, using immunoprecipitation and Western blot analysis, for expression of c-Met. Additionally, 16 separate specimens of malignant melanomas (MMs), 16 squamous cell carcinomas (SCCs), 16 basal cell carcinomas (BCCs) and 16 normal tissues were analyzed for the expression of c-Met using immunohistochemical studies.
RESULTS
Based on cultured cell immunoprecipitation and Western blot analysis, both A431 cells and G361 cells expressed c-Met, however, c-Met was expressed substantially more in G361 cells. Immunohistochemical examination of c-Met showed that it was over-expressed in all malignant skin cancers. In addition, c-Met expression was more increased in MM compared to other cancers.
CONCLUSION
In our study, c-Met is involved in malignant skin cancer development and the level of its expression is thought to be related to the degree of malignancy in melanoma cancers.
MeSH Terms
Figure
Reference
-
1. Wolf HK, Zarnegar R, Michalopoulos GK. Localization of hepatocyte growth factor in human and rat tissues: an immunohistochemical study. Hepatology. 1991. 14:488–494.
Article2. Wang Y, Selden AC, Morgan N, Stamp GW, Hodgson HJ. Hepatocyte growth factor/scatter factor expression in human mammary epithelium. Am J Pathol. 1994. 144:675–682.3. Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology. 1992. 15:149–155.
Article4. Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006. 12:3657–3660.
Article5. Pisters LL, Troncoso P, Zhau HE, Li W, von Eschenbach AC, Chung LW. c-Met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995. 154:293–298.
Article6. Matteucci E, Bendinelli P, Desiderio MA. Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis. 2009. 30:937–945.
Article7. Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, et al. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008. 283:4344–4351.
Article8. Rong S, Jeffers M, Resau JH, Tsarfaty I, Oskarsson M, Vande Woude GF. Met expression and sarcoma tumorigenicity. Cancer Res. 1993. 53:5355–5360.9. Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM. The receptor encoded by the human c-Met oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991. 49:323–328.
Article10. Sheth PR, Watowich SJ. Biochemical ground-rules regulating c-Met receptor tyrosine kinase activation and signaling. Cancer Ther. 2006. 4:1–12.11. Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984. 311:29–33.
Article12. Kuniyasu H, Yasui W, Yokozaki H, Kitadai Y, Tahara E. Aberrant expression of c-met mRNA in human gastric carcinomas. Int J Cancer. 1993. 55:72–75.
Article13. Natali PG, Nicotra MR, Di Renzo MF, Prat M, Bigotti A, Cavaliere R, et al. Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993. 68:746–750.
Article14. Di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, et al. Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer. 1994. 58:658–662.15. Boix L, Rosa JL, Ventura F, Castells A, Bruix J, Rodés J, et al. c-met mRNA overexpression in human hepatocellular carcinoma. Hepatology. 1994. 19:88–91.
Article16. Belfiore A, Gangemi P, Costantino A, Russo G, Santonocito GM, Ippolito O, et al. Negative/low expression of the Met/hepatocyte growth factor receptor identifies papillary thyroid carcinomas with high risk of distant metastases. J Clin Endocrinol Metab. 1997. 82:2322–2328.
Article17. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008. 7:504–516.
Article18. Pisters LL, el-Naggar AK, Luo W, Malpica A, Lin SH. c-Met proto-oncogene expression in benign and malignant human renal tissues. J Urol. 1997. 158:724–728.
Article19. Natali PG, Prat M, Nicotra MR, Bigotti A, Olivero M, Comoglio PM, et al. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer. 1996. 69:212–217.
Article20. Wagatsuma S, Konno R, Sato S, Yajima A. Tumor angiogenesis, hepatocyte growth factor, and c-Met expression in endometrial carcinoma. Cancer. 1998. 82:520–530.
Article21. Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, et al. Hepatocyte growth factor and its receptor (c-Met) in prostatic carcinoma. Am J Pathol. 1995. 147:386–396.22. Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology. 1997. 25:862–866.
Article23. Berchuck A, Soisson AP, Clarke-Pearson DL, Soper JT, Boyer CM, Kinney RB, et al. Immunohistochemical expression of CA 125 in endometrial adenocarcinoma: correlation of antigen expression with metastatic potential. Cancer Res. 1989. 49:2091–2095.24. Aasmundstad TA, Haugen OA, Johannesen E, Høe AL, Kvinnsland S. Oestrogen receptor analysis: correlation between enzyme immunoassay and immunohistochemical methods. J Clin Pathol. 1992. 45:125–129.
Article25. Ersoy B, Ozbilgin K, Kasirga E, Inan S, Coskun S, Tuglu I. Effect of growth hormone on small intestinal homeostasis relation to cellular mediators IGF-I and IGFBP-3. World J Gastroenterol. 2009. 15:5418–5424.
Article26. Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003. 22:309–325.27. Duh FM, Scherer SW, Tsui LC, Lerman MI, Zbar B, Schmidt L. Gene structure of the human MET proto-oncogene. Oncogene. 1997. 15:1583–1586.
Article28. Boccaccio C, Comoglio PM. Invasive growth: a Met-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006. 6:637–645.
Article29. Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993. 1155:357–371.
Article30. Chung LW, Li W, Gleave ME, Hsieh JT, Wu HC, Sikes RA, et al. Human prostate cancer model: roles of growth factors and extracellular matrices. J Cell Biochem Suppl. 1992. 16H:99–105.
Article31. Scarpino S, Stoppacciaro A, Colarossi C, Cancellario F, Marzullo A, Marchesi M, et al. Hepatocyte growth factor (HGF) stimulates tumour invasiveness in papillary carcinoma of the thyroid. J Pathol. 1999. 189:570–575.
Article32. Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene. 2008. 27:684–693.
Article33. Beviglia L, Matsumoto K, Lin CS, Ziober BL, Kramer RH. Expression of the c-Met/HGF receptor in human breast carcinoma: correlation with tumor progression. Int J Cancer. 1997. 74:301–309.
Article34. Lamszus K, Jin L, Fuchs A, Shi E, Chowdhury S, Yao Y, et al. Scatter factor stimulates tumor growth and tumor angiogenesis in human breast cancers in the mammary fat pads of nude mice. Lab Invest. 1997. 76:339–353.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of long interspersed element-1 open reading frame 1 and c-Met proto-oncogene protein expression in primary and recurrent colorectal cancers
- Expression of c-Met in ovarian epithelial tumor
- Fibroblast Growth Factor 4(FGF4) Expression in Malignant Skin Cancers
- Study of the Expression of E-cadherin, beta-catenin, and c-Met in Gastric Adenocarcinomas
- The Expression of High Mobility Group I (Y) Proteins in Human Breast Cancer