Ann Dermatol.
2013 May;25(2):173-180. 10.5021/ad.2013.25.2.173.
Potential Immunoinflammatory Role of Staphylococcal Enterotoxin A in Atopic Dermatitis: Immunohistopathological Analysis and in vitro Assay
- Affiliations
-
- 1Department of Veterinary Internal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea.
- 2Department of Microbiology, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea. kimdw@knu.ac.kr
- 4Department of Dermatology, Pusan National University School of Medicine, Busan, Korea.
- 5Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2171785
- DOI: http://doi.org/10.5021/ad.2013.25.2.173
Abstract
- BACKGROUND
The underlying mechanism of atopic dermatitis (AD) exacerbated by Staphylococcus aureus has not been established. However, we demonstrated recently that the majority of S. aureus strains colonized in the skin of Korean AD patients carried genes encoding staphylococcal enterotoxin A (SEA) and/or toxic shock syndrome toxin-1 (TSST-1).
OBJECTIVE
To clarify the role of staphylococcal superantigen, SEA in AD.
METHODS
With the lesional skin of 9 AD patients and normal looking skin of one healthy adult, we examined first the expression of SEA, staphylococcal enterotoxin B (SEB), and TSST-1 using immunohistochemical analysis. In addition, we investigated the effects of SEA on the expression of inflammation-related adhesion molecules and cytokines in human HaCaT keratinocytes and Human Umbilical Vein Endothelial Cells (HUVECs) by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and enzyme-linked immunosorbent assay.
RESULTS
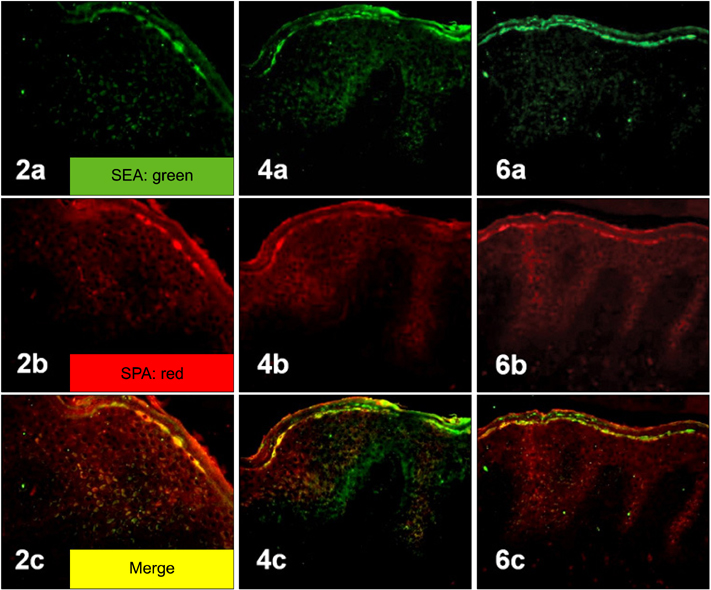
Staphylococcal protein A (SPA) and SEA were detected with increased immunoreactivity in AD patients. However, TSST-1 showed mild-to-moderate immunoreactivity in AD patients, whereas SEB was minimally detected. In the double immunofluorescence investigation, SEA and SPA were well co-localized. SEA induced upregulation of adhesion molecules and elicited inflammatory responses in HaCaT keratinocytes and HUVECs.
CONCLUSION
This study demonstrates the importance of SEA as an immunoinflammatory triggering factor of AD in Koreans.
MeSH Terms
-
Adult
Bacterial Toxins
Colon
Cytokines
Dermatitis, Atopic
Enterotoxins
Fluorescent Antibody Technique
Human Umbilical Vein Endothelial Cells
Humans
Keratinocytes
Shock, Septic
Skin
Staphylococcal Protein A
Staphylococcus aureus
Superantigens
Up-Regulation
Bacterial Toxins
Cytokines
Enterotoxins
Staphylococcal Protein A
Superantigens
Figure
Reference
-
1. Ricci G, Patrizi A, Neri I, Bendandi B, Masi M. Frequency and clinical role of Staphylococcus aureus overinfection in atopic dermatitis in children. Pediatr Dermatol. 2003. 20:389–392.
Article2. Hauser C, Wuethrich B, Matter L, Wilhelm JA, Sonnabend W, Schopfer K. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatologica. 1985. 170:35–39.3. Hanifin JM, Rogge JL. Staphylococcal infections in patients with atopic dermatitis. Arch Dermatol. 1977. 113:1383–1386.
Article4. Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977. 113:780–782.
Article5. Kim KH, Han JH, Chung JH, Cho KH, Eun HC. Role of staphylococcal superantigen in atopic dermatitis: influence on keratinocytes. J Korean Med Sci. 2006. 21:315–323.
Article6. Kim DW, Park JY, Park KD, Kim TH, Lee WJ, Lee SJ, et al. Are there predominant strains and toxins of Staphylococcus aureus in atopic dermatitis patients? Genotypic characterization and toxin determination of aureus isolated in adolescent and adult patients with atopic dermatitis. J Dermatol. 2009. 36:75–81.
Article7. Kim BS, Kim JY, Lim HJ, Lee WJ, Lee SJ, Kim JM, et al. Colonizing features of Staphylococcus aureus in early childhood atopic dermatitis and in mothers: a cross-sectional comparative study done at four kindergartens in Daegu, South Korea. Ann Allergy Asthma Immunol. 2011. 106:323–329.
Article8. Jiang XY, Wang CF, Wang CF, Zhang PJ, He ZY. Cloning and expression of Mycobacterium bovis secreted protein MPB83 in Escherichia coli. J Biochem Mol Biol. 2006. 39:22–25.
Article9. DeDent AC, McAdow M, Schneewind O. Distribution of protein A on the surface of Staphylococcus aureus. J Bacteriol. 2007. 189:4473–4484.
Article10. Leung DYM, Eichenfield LF, Boguniewicz M. Wolff K, Goldsmith LA, Katz Sl, Gilchrest BA, Paller AS, Leffell DJ, editors. Atopic dermatitis (Atopic eczema). Fitzpatrick's dermatology in general medicine. 2008. Vol. 2:7th ed. New York: McGraw-Hill Medical Cop.;146–158.11. Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002. 88:1–12.
Article12. Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008. 32:1405–1411.
Article13. Matsunaga T, Katayama I, Yokozeki H, Nishioka K. Superantigen-induced cytokine expression in organ-cultured human skin. J Dermatol Sci. 1996. 11:104–110.
Article14. Saloga J, Leung DY, Reardon C, Giorno RC, Born W, Gelfand EW. Cutaneous exposure to the superantigen staphylococcal enterotoxin B elicits a T-cell-dependent inflammatory response. J Invest Dermatol. 1996. 106:982–988.
Article15. Pivarcsi A, Gombert M, Dieu-Nosjean MC, Lauerma A, Kubitza R, Meller S, et al. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004. 173:5810–5817.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Specific Antibodies to Staphylococcal Enterotoxin B in Children with Atopic Dermatitis
- A Study on the Evaluation of the Staphylococcal Exotoxins and Staphylococcal Enterotoxin A-specific IgE Antibody in Childhood Atopic Dermatitis
- Association of the proliferation of CD4(+)/Vbeta17(+) cells and peripheral blood mononuclear cells in response to staphylococcal enterotoxin B(SEB) in atopic dermatitis
- Two Cases of Atopic Dermatitis Developing Ocular Complication and Immunological Disturbance
- A Comparison Study of the Staphylococcal Exotoxins and Staphylococcal Enterotoxin A-specific IgE Antibody between Childhood and Adulthood Atopic Dermatitis