Ann Dermatol.
2015 Oct;27(5):523-530. 10.5021/ad.2015.27.5.523.
Maintenance Therapy of Facial Seborrheic Dermatitis with 0.1% Tacrolimus Ointment
- Affiliations
-
- 1Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. dermap@hallym.or.kr
- 2Department of Dermatology, Pusan National University School of Medicine, Yangsan, Korea.
- 3Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea.
- 5Department of Dermatology, Korea University College of Medicine, Seoul, Korea.
- 6Department of Dermatology, Gachon University of Medicine and Science, Incheon, Korea.
- 7Department of Dermatology, Eulji University College of Medicine, Daejeon, Korea.
- 8Department of Dermatology, University of Ulsan College of Medicine, Seoul, Korea.
- 9Department of Dermatology, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 2171452
- DOI: http://doi.org/10.5021/ad.2015.27.5.523
Abstract
- BACKGROUND
Topical calcineurin inhibitors (TCIs) have been successfully used to treat seborrheic dermatitis (SD) patients. Meanwhile, treatment of atopic dermatitis (AD) with low-dose, intermittent TCI has been proved to reduce disease flare-ups. This regimen is known as a maintenance treatment.
OBJECTIVE
The aim of this trial was to investigate the efficacy and tolerability of a maintenance treatment with tacrolimus ointment in patients with facial SD.
METHODS
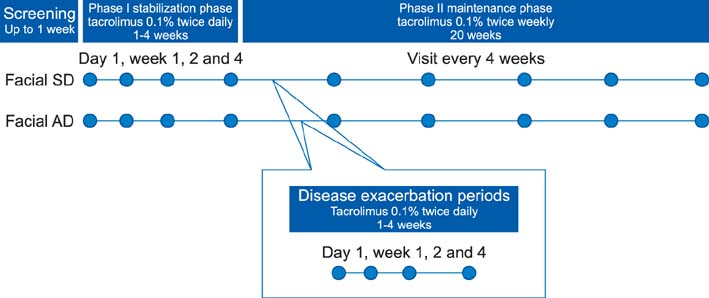
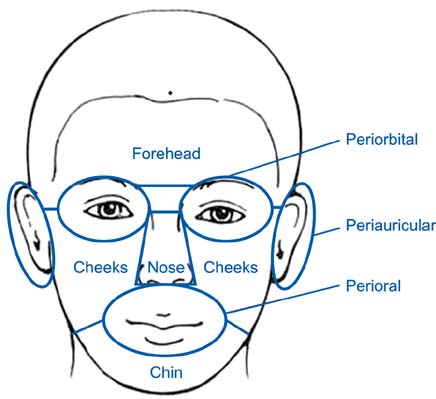
During the initial stabilization period, patients with facial SD or AD applied 0.1% tacrolimus ointment twice daily for up to 4 weeks. Clinical measurements were evaluated on either in the whole face or on separate facial regions. When an investigator global assessment score 1 was achieved, the patient applied tacrolimus twice weekly for 20 weeks. We also compared our results with recent published data of placebo controlled study to allow an estimation of the placebo effect.
RESULTS
The time to the first relapse during phase II was similar in both groups otherwise significantly longer than the placebo group. The recurrence-free curves of two groups were not significantly different from each other; otherwise the curve of the placebo group was significantly different. There were no significant differences between the 2 groups in the number of DEs, and treatment days for disease exacerbations (DEs). The adverse event profile was also similar between the 2 groups. During the 20 weeks of treatment, the study population tolerated tacrolimus ointment well.
CONCLUSION
The results of this study suggest that maintenance treatment with tacrolimus may be effective in preventing the occurrence of facial SD exacerbations.
MeSH Terms
Figure
Reference
-
1. Aschoff R, Kempter W, Meurer M. Seborrheic dermatitis. Hautarzt. 2011; 62:297–307.2. Cook BA, Warshaw EM. Role of topical calcineurin inhibitors in the treatment of seborrheic dermatitis: a review of pathophysiology, safety, and efficacy. Am J Clin Dermatol. 2009; 10:103–118.
Article3. Faergemann J, Bergbrant IM, Dohsé M, Scott A, Westgate G. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001; 144:549–556.
Article4. Bikowski J. Facial seborrheic dermatitis: a report on current status and therapeutic horizons. J Drugs Dermatol. 2009; 8:125–133.5. Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2004; 5:417–422.
Article6. Shemer A, Kaplan B, Nathansohn N, Grunwald MH, Amichai B, Trau H. Treatment of moderate to severe facial seborrheic dermatitis with itraconazole: an open non-comparative study. Isr Med Assoc J. 2008; 10:417–418.7. Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009; 60:125–136.
Article8. Kolmer HL, Taketomi EA, Hazen KC, Hughs E, Wilson BB, Platts-Mills TA. Effect of combined antibacterial and antifungal treatment in severe atopic dermatitis. J Allergy Clin Immunol. 1996; 98:702–707.
Article9. Rigopoulos D, Ioannides D, Kalogeromitros D, Gregoriou S, Katsambas A. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis A randomized open-label clinical trial. Br J Dermatol. 2004; 151:1071–1075.
Article10. Anstey A. Management of atopic dermatitis: nonadherence to topical therapies in treatment of skin disease and the use of calcineurin inhibitors in difficult eczema. Br J Dermatol. 2009; 161:219–220.
Article11. Tatlican S, Eren C, Oktay B, Eskioglu F, Durmazlar PK. Experience with repetitive use of pimecrolimus: exploring the effective and safe use in the treatment of relapsing seborrheic dermatitis. J Dermatolog Treat. 2010; 21:83–85.
Article12. Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011; 164:415–428.
Article13. Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. European Tacrolimus Ointment Study Group. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008; 63:742–750.
Article14. Breneman D, Fleischer AB Jr, Abramovits W, Zeichner J, Gold MH, Kirsner RS, et al. Tacrolimus Ointment Study Group. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008; 58:990–999.
Article15. Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. Br J Dermatol. 2009; 160:415–422.
Article16. Gupta G, Mallefet P, Kress DW, Sergeant A. Adherence to topical dermatological therapy: lessons from oral drug treatment. Br J Dermatol. 2009; 161:221–227.
Article17. Papp KA, Papp A, Dahmer B, Clark CS. Single-blind, randomized controlled trial evaluating the treatment of facial seborrheic dermatitis with hydrocortisone 1% ointment compared with tacrolimus 0.1% ointment in adults. J Am Acad Dermatol. 2012; 67:e11–e15.
Article18. Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. European Tacrolimus Ointment Study Group. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008; 159:1348–1356.
Article19. Wollenberg A, Ehmann LM. Long term treatment concepts and proactive therapy for atopic eczema. Ann Dermatol. 2012; 24:253–260.
Article20. Luger T, Paul C. Potential new indications of topical calcineurin inhibitors. Dermatology. 2007; 215:Suppl 1. 45–54.
Article21. Kim BS, Kim SH, Kim MB, Oh CK, Jang HS, Kwon KS. Treatment of facial seborrheic dermatitis with pimecrolimus cream 1%: an open-label clinical study in Korean patients. J Korean Med Sci. 2007; 22:868–872.
Article22. Ozden MG, Tekin NS, Ilter N, Ankarali H. Topical pimecrolimus 1% cream for resistant seborrheic dermatitis of the face: an open-label study. Am J Clin Dermatol. 2010; 11:51–54.
Article23. Meshkinpour A, Sun J, Weinstein G. An open pilot study using tacrolimus ointment in the treatment of seborrheic dermatitis. J Am Acad Dermatol. 2003; 49:145–147.
Article24. Kim TW, Mun JH, Jwa SW, Song M, Kim HS, Ko HC, et al. Proactive treatment of adult facial seborrhoeic dermatitis with 0.1% tacrolimus ointment: randomized, double-blind, vehicle-controlled, multi-centre trial. Acta Derm Venereol. 2013; 93:557–561.
Article25. Fukuie T, Nomura I, Horimukai K, Manki A, Masuko I, Futamura M, et al. Proactive treatment appears to decrease serum immunoglobulin-E levels in patients with severe atopic dermatitis. Br J Dermatol. 2010; 163:1127–1129.
Article26. Poole CD, Chambers C, Sidhu MK, Currie CJ. Health-related utility among adults with atopic dermatitis treated with 01 tacrolimus ointment as maintenance therapy over the long term: findings from the Protopic CONTROL study. Br J Dermatol. 2009; 161:1335–1340.
Article27. Sugita T, Tajima M, Tsubuku H, Tsuboi R, Nishikawa A. A new calcineurin inhibitor, pimecrolimus, inhibits the growth of Malassezia spp. Antimicrob Agents Chemother. 2006; 50:2897–2898.
Article28. Sugita T, Tajima M, Ito T, Saito M, Tsuboi R, Nishikawa A. Antifungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J Clin Microbiol. 2005; 43:2824–2829.
Article29. Braza TJ, DiCarlo JB, Soon SL, McCall CO. Tacrolimus 0.1% ointment for seborrhoeic dermatitis: an open-label pilot study. Br J Dermatol. 2003; 148:1242–1244.
Article30. Paller AS, Lebwohl M, Fleischer AB Jr, Antaya R, Langley RG, Kirsner RS, et al. US/Canada Tacrolimus Ointment Study Group. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. J Am Acad Dermatol. 2005; 52:810–822.
Article31. Fleischer AB Jr, Abramovits W, Breneman D, Jaracz E. US/Canada Tacrolimus Ointment Study Group. Tacrolimus ointment is more effective than pimecrolimus cream in adult patients with moderate to very severe atopic dermatitis. J Dermatolog Treat. 2007; 18:151–157.
Article32. Yin ZQ, Zhang WM, Song GX, Luo D. Meta-analysis on the comparison between two topical calcineurin inhibitors in atopic dermatitis. J Dermatol. 2012; 39:520–526.
Article33. Abramovits W, Fleischer AB Jr, Jaracz E, Breneman D. Adult patients with moderate atopic dermatitis: tacrolimus ointment versus pimecrolimus cream. J Drugs Dermatol. 2008; 7:1153–1158.34. Kempers S, Boguniewicz M, Carter E, Jarratt M, Pariser D, Stewart D, et al. A randomized investigator-blinded study comparing pimecrolimus cream 1% with tacrolimus ointment 0.03% in the treatment of pediatric patients with moderate atopic dermatitis. J Am Acad Dermatol. 2004; 51:515–525.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tacrolimus ointment: An Open study for Effects on Severe Facial Atopic Dermatitis in Korean
- Tinea Incognito Caused by Application of 0.03% Tacrolimus (Protopic(R)) Ointment in Atopic Dermatitis Patient
- Four Cases of Atopic Dermatitis with Topical Tacrolimus Therapy
- The Efficacy of Tapering Treatment with 0.1% Tacrolimus Ointment in Adult Patients with Atopic Dermatitis in Korea
- Granulomatous Perioral Dermatitis Presented with Facial Eczematous Lesion