Infect Chemother.
2011 Dec;43(6):458-467. 10.3947/ic.2011.43.6.458.
Trend of Bacterial Resistance for the Past 50 Years in Korea and Future Perspectives - Gram-negative Bacteria
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. leekcp@yuhs.ac
- 2Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2170357
- DOI: http://doi.org/10.3947/ic.2011.43.6.458
Abstract
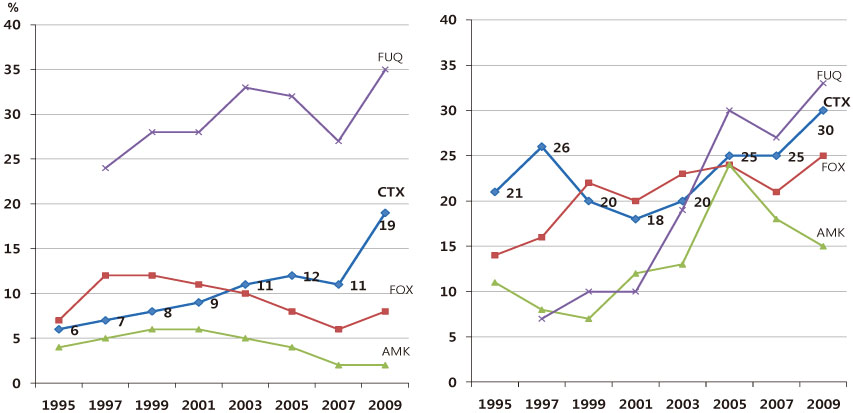
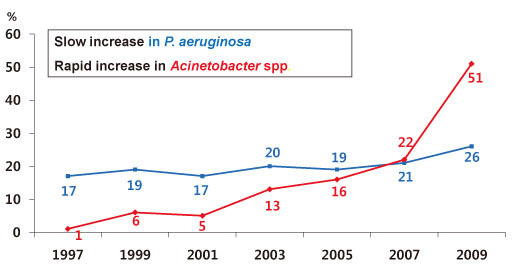
- The increasing prevalence of antimicrobial-resistant bacteria has become a serious problem in many parts of the world, including Korea. The resistance of of Gram-positive cocci was once considered to be a more serious problem, but the recent emergence of multi-resistant Gram-negative bacilli has raised great concerns. In Korea, the prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae and fluoroquinolone-resistant E. coli, K. pneumoniae, Acinetobacter spp., and Pseudomonas aeruginosa has gradually increased. The increase in imipenem resistance was slight in P. aeruginosa, but drastic in Acinetobacter spp. It is certain that problematic antimicrobial-organism combinations are prevalent in Korea, increase of resistant bacteria will continue in the future. The development of new antimicrobial agents is considered difficult. Therefore, it is very important to use existing antimicrobial agents prudently, to extend the efficacy, to prevent infections, and tostrengthen infection control measures to prevent spread of resistant bacteria.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Changing Patterns of Antibiotics Usage in Korea during 1981-2008
Youn Jeong Kim, Hyun Ji Chun, Jung Woo Lee, Kyung-Wook Hong, Sang Il Kim, Seong Heon Wie, Yang Ree Kim, Moon Won Kang
Infect Chemother. 2012;44(6):411-418. doi: 10.3947/ic.2012.44.6.411.
Reference
-
1. Brötz-Oesterhelt H, Sass P. Postgenomic strategies in antibacterial drug discovery. Future Microbiol. 2010. 5:1553–1579.
Article2. Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011. 52:879–891.
Article3. Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van PH. ANSORP Study Group. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011. 66:1061–1069.
Article4. Chong Y, Yi KN, Chang DK, Lee SY. Quality control of antibiotic susceptibility testing. J Korean Med Assoc. 1977. 20:433–440.5. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests-proposed standard. NCCLS document. 1975. Villanova, Pa.: NCCLS.6. Park SH. Pathogenic bacteria isolated from Korean patients. Korean J Infect Dis. 1969. 1:33–48.7. Park SH. Antibiotic susceptibility of pathogenic microorganisms isolated in 1969. J Korean Med Assoc. 1970. 13:71–80.8. Park SJ, Chong Y, Lee SY. Antibiotic susceptibility of clinical isolates of bacteria. Korean J Pathol. 1977. 11:119–125.9. Chung HY. The changing pattern of antimicrobial susceptibility of hospital isolates. Korean J Infect Dis. 1986. 18:1–10.10. Chong Y. Trend of antimicrobial agent resistance of bacteria isolated from clinical materials. Korean J Infect Dis. 1989. 21:243–255.11. Lee K, Chong Y, Kwon OH, Park HS, Kim JM. Antimicrobial susceptibilities of bacteria isolated during 1988-1992. J Korean Soc Chemother. 1993. 158–168.12. Shah PM, Stille W. Escherichia coli and Klebsiella pneumoniae strains more susceptible to cefoxitin than to third generation cephalosporins. J Antimicrob Chemother. 1983. 11:597–598.
Article13. Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001. 14:933–951.
Article14. Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005. 18:657–686.
Article15. Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004. 48:1–14.
Article16. Lee K, Cho SR, Lee CS, Chong Y, Kwon OH. Prevalence of extended broad-spectrum β-lactamase in Escherichia coli and Klebsiella pneumoniae. Korean J Infect Dis. 1994. 26:341–348.17. Chong Y, Lee K, Park YJ, Jeon DS, Lee MH, Kim MY, Chang CH, Kim EC, Lee NY, Kim HS, Kang ES, Cho HC, Paik IK, Lee HS, Jang SJ, Park AJ, Cha YJ, Kang SH, Lee MH, Song W, Shin JH. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 1998. 39:569–577.
Article18. Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, Park YJ, Yong D, Jeong SH, Chong Y. KONSAR Group. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011. 52:793–802.
Article19. Chong Y, Lee K, Okamoto R, Inoue M. Characteristics of extended-spectrum β-lactam hydrolyzing activity of Klebsiella pneumoniae and Escherichia coli strains isolated from clinical specimens. Korean J Infect Dis. 1997. 29:477–485.20. Pai H, Kim JM, Kwon YM, Lee K, Chong Y, Kim EC, Cho DT. Characterization of extended spectrum β-lactamase in Klebsiella pneumoniae isolated in Korea. Korean J Infect Dis. 1997. 29:93–103.21. Kim J, Kwon Y, Pai H, Kim JW, Cho DT. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998. 36:1446–1449.
Article22. Pai H, Lyu S, Lee JH, Kim J, Kwon Y, Kim JW, Choe KW. Survey of extended-spectrum β-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea. J Clin Microbiol. 1999. 37:1758–1763.
Article23. Lee K, Yong D, Yum JH, Kim HH, Chong Y. Diversity of TEM-52 extended-spectrum β-lactamase-producing non-typhoidal Salmonella isolates in Korea. J Antimicrob Chemother. 2003. 52:493–496.
Article24. Pai H, Choi EH, Lee HJ, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001. 39:3747–3749.
Article25. Jeong SH, Bae IK, Kwon SB, Lee JH, Jung HI, Song JS, Jeong BC, Kim SJ, Lee SH. Investigation of extended-spectrum β-lactamases produced by clinical isolates of Klebsiella pneumoniae and Escherichia coli in Korea. Lett Appl Microbiol. 2004. 39:41–47.
Article26. Pai H, Kim MR, Seo MR, Choi TY, Oh SH. A nosocomial outbreak of Escherichia coli producing CTX-M-15 and OXA-30 β-lactamase. Infect Control Hosp Epidemiol. 2006. 27:312–314.
Article27. Park YJ, Park SY, Oh EJ, Park JJ, Lee KY, Woo GJ, Lee K. Occurrence of extended-spectrum β-lactamases among chromosomal AmpC-producing Enterobacter cloacae, Citrobacter freundii, and Serratia marcescens in Korea and investigation of screening criteria. Diagn Microbiol Infect Dis. 2005. 51:265–269.
Article28. Park YJ, Lee S, Kim YR, Oh EJ, Woo GJ, Lee K. Occurrence of extended-spectrum β-lactamases and plasmid-mediated AmpC β-lactamases among Korean isolates of Proteus mirabilis. J Antimicrob Chemother. 2006. 57:156–158.
Article29. Song W, Lee H, Lee K, Jeong SH, Bae IK, Kim JS, Kwak HS. CTX-M-14 and CTX-M-15 enzymes are the dominant type of extended-spectrum β-lactamase in clinical isolates of Escherichia coli from Korea. J Med Microbiol. 2009. 58:261–266.
Article30. Song W, Kim J, Bae IK, Jeong SH, Seo YH, Shin JH, Jang SJ, Uh Y, Shin JH, Lee MK, Lee K. Chromosome-encoded AmpC and CTX-M extended-spectrum β-lactamases in clinical isolates of Proteus mirabilis from Korea. Antimicrob Agents Chemother. 2011. 55:1414–1419.
Article31. Lee K, Chong Y, Yong D, Yum JH, Chong S. Evolution of resistance and spread of multidrug-resistant bacteria. 2007. Seoul: Seo Heung Publishing co..32. Pai H, Hong JY, Byeon JH, Kim YK, Lee HJ. High prevalence of extended-spectrum β-lactamase-producing strains among blood isolates of Enterobacter spp. collected in a tertiary hospital during an 8-year period and their antimicrobial susceptibility patterns. Antimicrob Agents Chemother. 2004. 48:3159–3161.
Article33. Kim J, Lim YM. Prevalence of derepressed ampC mutants and extended-spectrum β-lactamase producers among clinical isolates of Citrobacter freundii, Enterobacter spp., and Serratia marcescens in Korea: dissemination of CTX-M-3, TEM-52, and SHV-12. J Clin Microbiol. 2005. 43:2452–2455.
Article34. Bauernfeind A, Chong Y, Schweighart S. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989. 17:316–321.
Article35. Papanicolaou GA, Medeiros AA, Jacoby GA. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino-and alpha-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1990. 34:2200–2209.
Article36. Fosberry AP, Payne DJ, Lawlor EJ, Hodgson JE. Cloning and sequence analysis of blaBIL-1, a plasmid-mediated class C β-lactamase gene in Escherichia coli BS. Antimicrob Agents Chemother. 1994. 38:1182–1185.
Article37. Gonzalez Leiza M, Perez-Diaz JC, Ayala J, Casellas JM, Martinez-Beltran J, Bush K, Baquero F. Gene sequence and biochemical characterization of FOX-1 from Klebsiella pneumoniae, a new AmpC-type plasmid-mediated β-lactamase with two molecular variants. Antimicrob Agents Chemother. 1994. 38:2150–2157.
Article38. Horii T, Arakawa Y, Ohta M, Sugiyama T, Wacharotayankun R, Ito H, Kato N. Characterization of a plasmid-borne and constitutively expressed blaMOX-1 gene encoding AmpC-type β-lactamase. Gene. 1994. 139:93–98.
Article39. Barnaud G, Arlet G, Verdet C, Gaillot O, Lagrange PH, Philippon A. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob Agents Chemother. 1998. 42:2352–2358.
Article40. Tzouvelekis LS, Tzelepi E, Mentis AF, Tsakris A. Identification of a novel plasmid-mediated β-lactamase with chromosomal cephalosporinase characteristics from Klebsiella pneumoniae. J Antimicrob Chemother. 1993. 31:645–654.
Article41. Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the foss of an outer membrane protein. Antimicrob Agents Chemother. 1997. 41:563–569.
Article42. Bauernfeind A, Schneider I, Jungwirth R, Sahly H, Ullmann U. A novel type of AmpC β-lactamase, ACC-1, produced by a Klebsiella pneumoniae strain causing nosocomial pneumonia. Antimicrob Agents Chemother. 1999. 43:1924–1931.
Article43. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, Yong D, Chong Y. Prevalence of plasmid-mediated AmpC β-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb Drug Resist. 2006. 12:44–49.
Article44. Yoo JS, Byeon J, Yang J, Yoo JI, Chung GT, Lee YS. High prevalence of extended-spectrum β-lactamases and plasmid-mediated AmpC β-lactamases in Enterobacteriaceae isolated from long-term care facilities in Korea. Diagn Microbiol Infect Dis. 2010. 67:261–265.
Article45. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011. 55:4943–4960.
Article46. Lee K, Yong D, Choi YS, Yum JH, Kim JM, Woodford N, Livermore DM, Chong Y. Reduced imipenem susceptibility in Klebsiella pneumoniae clinical isolates with plasmid-mediated CMY-2 and DHA-1 β-lactamases co-mediated by porin loss. Int J Antimicrob Agents. 2007. 29:201–206.
Article47. Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007. 20:440–458.
Article48. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001. 45:1151–1161.
Article49. Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991. 35:147–151.
Article50. Donald HM, Scaife W, Amyes SG, Young HK. Sequence analysis of ARI-1, a novel OXA β-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob Agents Chemother. 2000. 44:196–199.
Article51. Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006. 57:373–383.
Article52. Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005. 18:306–325.
Article53. Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, Rossolini GM, Chong Y. Novel acquired metallo-β-lactamase gene, blaSIM-1, n a class integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005. 49:4485–4491.
Article54. Gupta V. Metallo β-lactamases in Pseudomonas aeruginosa and Acinetobacter species. Expert Opin Investig Drugs. 2008. 17:131–143.55. Poirel L, Rodríguez-Martínez JM, Al Naiemi N, Debets-Ossenkopp YJ, Nordmann P. Characterization of DIM-1, an integron-encoded metallo-β-lactamase from a Pseudomonas stutzeri clinical isolate in the Netherlands. Antimicrob Agents Chemother. 2010. 54:2420–2424.
Article56. Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009. 53:5046–5054.
Article57. Sekiguchi J, Morita K, Kitao T, Watanabe N, Okazaki M, Miyoshi-Akiyama T, Kanamori M, Kirikae T. KHM-1, a novel plasmid-mediated metallo-β-lactamase from a Citrobacter freundii clinical isolate. Antimicrob Agents Chemother. 2008. 52:4194–4197.
Article58. Lee K, Lim JB, Yum JH, Yong D, Chong Y, Kim JM, Livermore DM. blaVIM-2 cassettes-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob Agents Chemother. 2002. 46:1053–1058.
Article59. Yum JH, Yi K, Lee H, Yong D, Lee K, Kim JM, Rossolini GM, Chong Y. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two novel integrons carrying blaVIM-2 gene cassettes. J Antimicrob Chemother. 2002. 49:837–840.
Article60. Yum JH, Yong D, Lee K, Kim HS, Chong Y. A new integron carrying VIM-2 metallo-β-lactamase gene cassette in a Serratia marcescens isolate. Diagn Microbiol Infect Dis. 2002. 42:217–219.
Article61. Jeong SH, Lee K, Chong Y, Yum JH, Lee SH, Choi HJ, Kim JM, Park KH, Han BH, Lee SW, Jeong TS. Characterization of a new integron containing VIM-2, a metallo-β-lactamase gene cassette, in a clinical isolate of Enterobacter cloacae. J Antimicrob Chemother. 2003. 51:397–400.
Article62. Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y. Korean Nationwide Surveillance of Antimicrobial Resistance Group. VIM- and IMP-type metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003. 9:868–871.
Article63. Ryoo NH, Lee K, Lim JB, Lee YH, Bae IK, Jeong SH. Outbreak by meropenem-resistant Pseudomonas aeruginosa producing IMP-6 metallo-β-lactamase in a Korean hospital. Diagn Microbiol Infect Dis. 2009. 63:115–117.
Article64. Lee K, Kim CK, Hong SG, Choi J, Song S, Koh E, Yong D, Jeong SH, Yum JH, Docquier JD, Rossolini GM, Chong Y. Characteristics of clinical isolates of Acinetobacter genomospecies 10 carrying two different metallo-β-lactamases. Int J Antimicrob Agents. 2010. 36:259–263.
Article65. Kim MN, An D, Chung HS, Yong D, Lee K, Chong Y. Characterization of NDM-1-producing Klebsiella pneumoniae isolated from four Korean patients within a month. In : C1-1222a, 51th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2011 September 17-20; Chicago.66. Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin Infect Dis. 2004. 39:55–60.
Article67. Hossain A, Ferraro MJ, Pino RM, Dew RB 3rd, Moland ES, Lockhart TJ, Thomson KS, Goering RV, Hanson ND. Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 in an Enterobacter sp. Antimicrob Agents Chemother. 2004. 48:4438–4440.
Article68. Villegas MV, Lolans K, Correa A, Suarez CJ, Lopez JA, Vallejo M, Quinn JP. Colombian Nosocomial Resistance Study Group. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006. 50:2880–2882.
Article69. Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumonia isolate from France. Antimicrob Agents Chemother. 2005. 49:4423–4424.
Article70. Rhee JY, Park YK, Shin JY, Choi JY, Lee MY, Peck KR, Song JH, Ko KS. KPC-producing extreme drug-resistant Klebsiella pneumoniae isolate from a patient with diabetes mellitus and chronic renal failure on hemodialysis in South Korea. Antimicrob Agents Chemother. 2010. 54:2278–2279.
Article71. Roh KH, Lee CK, Sohn JW, Song W, Yong D, Lee K. Isolation of a Klebsiella pneumoniae isolate of sequence type 258 producing KPC-2 carbapenemase in Korea. Korean J Lab Med. 2011. 31:298–301.
Article72. Yoo J, Kim H, Yang J, Chung G, Lee Y. Emergence of KPC-2 producing Klebsiella pneumoniae ST258 isolates from national surveillance for carbapenem-resistant Enterobacteriaceae (CRE) in Korea. In : C2-652, 51th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2011 September 17-20; Chicago.73. Afzal-Shah M, Woodford N, Livermore DM. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2001. 45:583–588.
Article74. Naas T, Poirel L, Nordmann P. Pyrosequencing for rapid identification of carbapenem-hydrolyzing OXA-type β-lactamases in Acinetobacter baumannii. Clin Microbiol Infect. 2006. 12:1236–1240.
Article75. Lee Y, Lee J, Jeong SH, Lee J, Bae IK, Lee K. Carbapenem-non-susceptible Acinetobacter baumannii of sequence type 92 or its single-locus variants with a G428T substitution in zone 2 of the rpoB gene. J Antimicrob Chemother. 2011. 66:66–72.
Article76. Lee K, Kim MN, Choi TY, Cho SE, Lee S, Whang DH, Yong D, Chong Y, Woodford N, Livermore DM. KONSAR Group. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009. 33:520–524.
Article77. Livermore DM, Hill RL, Thomson H, Charlett A, Turton JF, Pike R, Patel BC, Manuel R, Gillespie S, Balakrishnan I, Barrett SP, Cumberland N, Twagira M. C-MRAB Study Group. Antimicrobial treatment and clinical outcome for infections with carbapenem- and multiply-resistant Acinetobacter baumannii around London. Int J Antimicrob Agents. 2010. 35:19–24.
Article78. Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, Chung DR, Peck KR, Song JH. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007. 60:1163–1167.
Article79. Song W, Lee TJ, Park MJ, Kim HS, Kim JS, Woo HJ, Lee KM. Susceptibility of clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa to colistin and polymyxin B in Korea. Infect Chemother. 2006. 38:362–366.80. Kim CK, Lee Y, Lee H, Woo GJ, Song W, Kim MN, Lee WG, Jeong SH, Lee K, Chong Y. Prevalence and diversity of carbapenemases among imipenem-nonsusceptible Acinetobacter isolates in Korea: emergence of a novel OXA-182. Diagn Microbiol Infect Dis. 2010. 68:432–438.
Article81. Galimand M, Courvalin P, Lambert T. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother. 2003. 47:2565–2571.
Article82. Lee H, Yong D, Yum JH, Roh KH, Lee K, Yamane K, Arakawa Y, Chong Y. Dissemination of 16S rRNA methylase-mediated highly amikacin-resistant isolates of Klebsiella pneumoniae and Acinetobacter baumannii in Korea. Diagn Microbiol Infect Dis. 2006. 56:305–312.
Article83. Park YJ, Lee S, Yu JK, Woo GJ, Lee K, Arakawa Y. Co-production of 16S rRNA methylases and extended-spectrum β-lactamases in AmpC-producing Enterobacter cloacae, Citrobacter freundii and Serratia marcescens in Korea. J Antimicrob Chemother. 2006. 58:907–908.
Article84. Lai CC, Wang CY, Chu CC, Tan CK, Lu CL, Lee YC, Huang YT, Lee PI, Hsueh PR. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J Antimicrob Chemother. 2011. 66:1374–1382.
Article85. Kim SI. Trend of antimicrobial usage in Korea. 2011. In : 2011 KFDA International Symposium on Antimicrobial Resistance; Korea: YUHS.86. Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001. 7:337–341.
Article87. Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006. 6:629–640.
Article88. Jeong JY, Yoon HJ, Kim ES, Lee Y, Choi SH, Kim NJ, Woo JH, Kim YS. Detection of qnr in clinical isolates of Escherichia coli from Korea. Antimicrob Agents Chemother. 2005. 49:2522–2524.
Article89. Pai H, Seo MR, Choi TY. Association of QnrB determinants and production of extended-spectrum β-lactamases or plasmid-mediated AmpC β-lactamases in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2007. 51:366–368.
Article90. Park YJ, Yu JK, Lee S, Oh EJ, Woo GJ. Prevalence and diversity of qnr alleles in AmpC-producing Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii and Serratia marcescens: a multi-centre study from Korea. J Antimicrob Chemother. 2007. 60:868–871.
Article91. Jeong HS, Bae IK, Shin JH, Jung HJ, Kim SH, Lee JY, Oh SH, Kim HR, Chang CL, Kho WG, Lee JN. Prevalence of plasmid-mediated quinolone resistance and its association with extended-spectrum β-lactamase and AmpC β-lactamase in Enterobacteriaceae. Korean J Lab Med. 2011. 31:257–264.
Article92. Kallen AJ, Srinivasan A. Current epidemiology of multidrug-resistant gram-negative bacilli in the United States. Infect Control Hosp Epidemiol. 2010. 31:Suppl 1. S51–S54.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metallo-beta-lactamase Producing Gram-negative Bacilli
- Strategies to combat Gram-negative bacterial resistance to conventional antibacterial drugs: a review
- The Impact of the Antibiotic Burden on the Selection of its Resistance among Gram Negative Bacteria Isolated from Children
- Staphylococcus aureus Membrane Vesicles and Its Potential Role in Bacterial Pathogenesis
- Trend of Resistance to the Third Generation Cephalosporin of Gram Negative Bacteria in Patients with Spontaneous Bacterial Peritonitis