J Bacteriol Virol.
2010 Dec;40(4):199-206. 10.4167/jbv.2010.40.4.199.
Synthetic and Adenovirus Delivered Small Interference RNA Pools Targeting Conserved Regions of Foot-and-Mouth Disease Virus
- Affiliations
-
- 1Foreign Animal Disease Division, National Veterinary Research and Quarantine Service, Ministry for Food, Agriculture, Forestry and Fisheries, Anyang, Korea. parkjhvet@korea.kr
- 2Department of Veterinary Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 2168584
- DOI: http://doi.org/10.4167/jbv.2010.40.4.199
Abstract
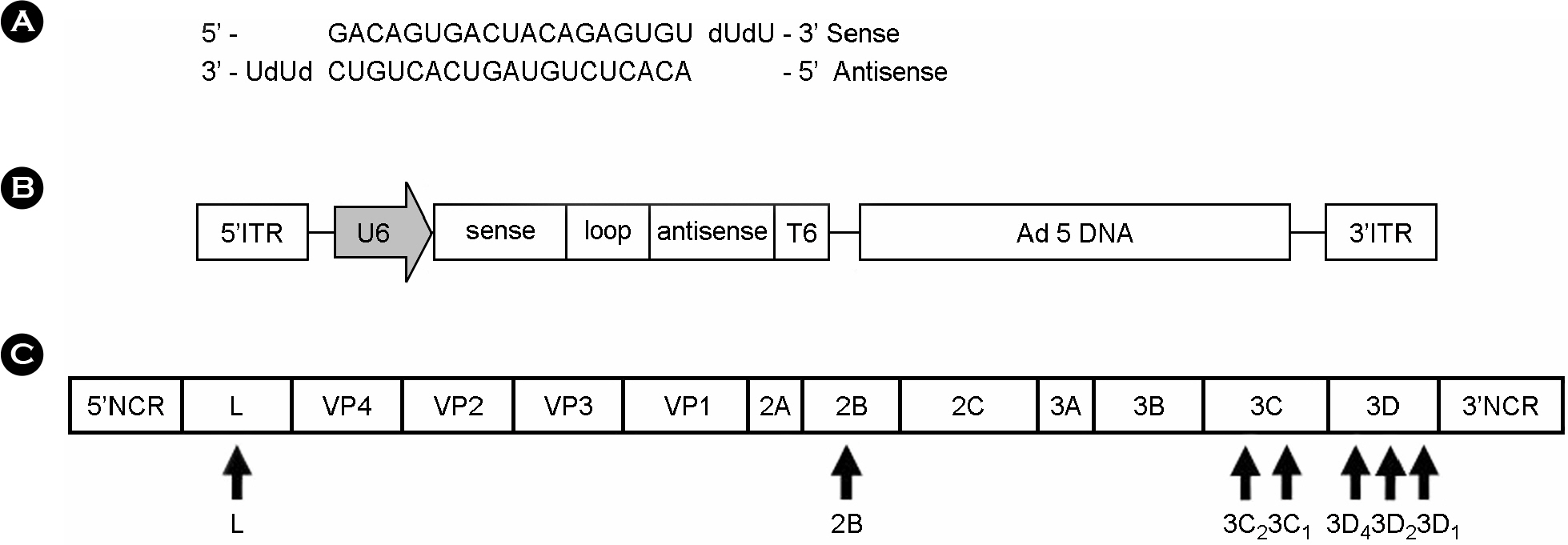
- Foot-and-mouth disease (FMD) is an economically significant animal disease because of the speed of its transmission. Routine vaccination may not be effective for early protection in an outbreak situation. Small interfering RNA (siRNA) can be used as a rapid, effective, and an alternative antiviral approach. In this study, we screened 15 synthetic siRNAs to inhibit FMD virus replication in IBRS-2 cells and selected 10 siRNA sequences. Furthermore, we produced 7 adenoviruses expressing shRNA targeting conserved regions of FMDV, such as a leader sequence and nonstructural protein regions, and showed their antiviral effects. We compared the antiviral effects among them and compared between synthetic siRNAs and adenovirus-delivered siRNAs. In particular, the most efficient siRNA, 3C2, was the conserved sequence in the O, A, Asia 1, and C serotypes of FMDV and was located in the predicted loop structure. The pool of sequences including 3C2 and recombinant adenoviruses could be applied for multiple siRNAs and protection in a broad range of cells and animals.
Keyword
MeSH Terms
Figure
Reference
-
1). Pereira HG. Foot-and-mouth disease animals. New York, NY: Academic Press. 1981. 333–63.2). Bachrach HL. Foot-and-mouth disease. Annu Rev Microbiol. 1968. 22:201–44.
Article3). Domingo E., Escarmís C., Baranowski E., Ruiz-Jarabo CM., Carrillo E., Núñez JI, et al. Evolution of foot-and-mouth disease virus. Virus Res. 2003. 91:47–63.
Article4). Knowles NJ., Samuel AR. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003. 91:65–80.
Article5). Gitlin L., Stone JK., Andino R. Poliovirus escape from RNA interference: short interfering RNA-target recognition and implications for therapeutic approaches. J Virol. 2005. 79:1027–35.
Article6). Saulnier A., Pelletier I., Labadie K., Colbère-Garapin F. Complete cure of persistent virus infections by antiviral iRNAs. Mol Ther. 2006. 13:142–50.7). Jacque JM., Triques K., Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002. 418:435–8.
Article8). Lee NS., Rossi JJ. Control of HIV-1 replication by RNA interference. Virus Res. 2004. 102:53–8.
Article9). Hamasaki K., Nakao K., Matsumoto K., Ichikawa T., Ishikawa H., Eguchi K. Short interfering RNA-directed inhibition of hepatitis B virus replication. FEBS Lett. 2003. 543:51–4.
Article10). Kahana R., Nakao K., Matsumoto K., Ichikawa T., Ishikawa H., Eguchi K. Inhibition of foot-and-mouth disease virus replication by small interfering RNA. J Gen Virol. 2004. 85:3213–7.
Article11). Chen W., Yan W., Du Q., Fei L., Liu M., Ni Z, et al. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J Virol. 2004. 78:6900–7.
Article12). Chen W., Liu M., Jiao Y., Yan W., Wei X., Chen J, et al. Adenovirus-mediated RNA interference against foot-and-mouth disease virus infection both in vitro and in vivo. J Virol. 2006. 80:3559–66.13). Souza AP., Haut L., Reyes-Sandoval A., Pinto AR. Recombinant viruses as vaccines against viral diseases. Braz J Med Biol Res. 2005. 38:509–22.
Article14). Reed LI., Muench H. A simple metheod of estimating fifty percent and points. Am J Hygiene. 1938. 27:493–7.15). Roeder PL., Le Blanc Smith PM. Detection and typing of foot-and-mouth disease virus by enzyme-linked immunosorbent assay: a sensitive, rapid and reliable technique for primary diagnosis. Res Vet Sci. 1987. 43:225–32.
Article16). Thompson JD., Higgins DG., Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994. 22:4673–80.
Article17). Hall TA. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999. 41:95–8.18). Root DE., Hacohen N., Hahn WC., Lander ES., Sabatini DM. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat Methods. 2006. 3:715–9.
Article19). Lo HL., Chang T., Yam P., Marcovecchio PM., Li S., Zaia JA, et al. Inhibition of HIV-1 replication with designed miRNAs expressed from RNA polymerase II promoters. Gene Ther. 2007. 14:1503–12.
Article20). Makinen PI., Koponen JK., Karkkainen AM., Malm TM., Pulkkinen KH., Koistinaho J, et al. Stable RNA interference: comparison of U6 and H1 promoters in endothelial cells and in mouse brain. J Gene Med. 2006. 8:433–41.21). Schubert S., Grunweller A., Erdmann VA., Kurreck J. Local RNA target structure influences siRNA efficacy: systematic analysis of intentionally designed binding regions. J Mol Biol. 2005. 348:883–93.
Article22). de Almeida RS., Keita D., Libeau G., Albina E. Structure and sequence motifs of siRNA linked with in vitro down-regulation of morbillivirus gene expression. Antiviral Res. 2008. 79:37–48.23). Lee HS., Ahn J., Jee Y., Seo IS., Jeon EJ., Jeon ES, et al. Universal and mutation-resistant anti-enteroviral activity: potency of small interfering RNA complementary to the conserved cis-acting replication element within the enterovirus coding region. J Gen Virol. 2007. 88:2003–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strategy for Novel Vaccine and Antivirals Against Foot-and-Mouth Disease
- Rescue of Synthetic Hantavirus-Like Foreign RNA by Hantavirus as a Helper Virus
- Inhibition of hepatitis B virus replication by RNA interference
- Development of synthetic peptide ELISA based on nonstructural protein 2C of foot and mouth disease virus
- Hand, Foot, and Mouth Disease: Clinical and Virological Investigations