J Bacteriol Virol.
2008 Mar;38(1):11-17. 10.4167/jbv.2008.38.1.11.
Identification of the Proteinase K-resistant Antigen of Orientia tsutsugamushi by Monoclonal Antibodies
- Affiliations
-
- 1Department of Microbiology, Center for Advanced Medical Education by BK21 Project, Inha University College of Medicine, Inchon, Korea. jaeskang@inha.ac.kr
- 2Research Institute for Medical Scineces, Inha University College of Medicine, Inchon, Korea.
- KMID: 2168514
- DOI: http://doi.org/10.4167/jbv.2008.38.1.11
Abstract
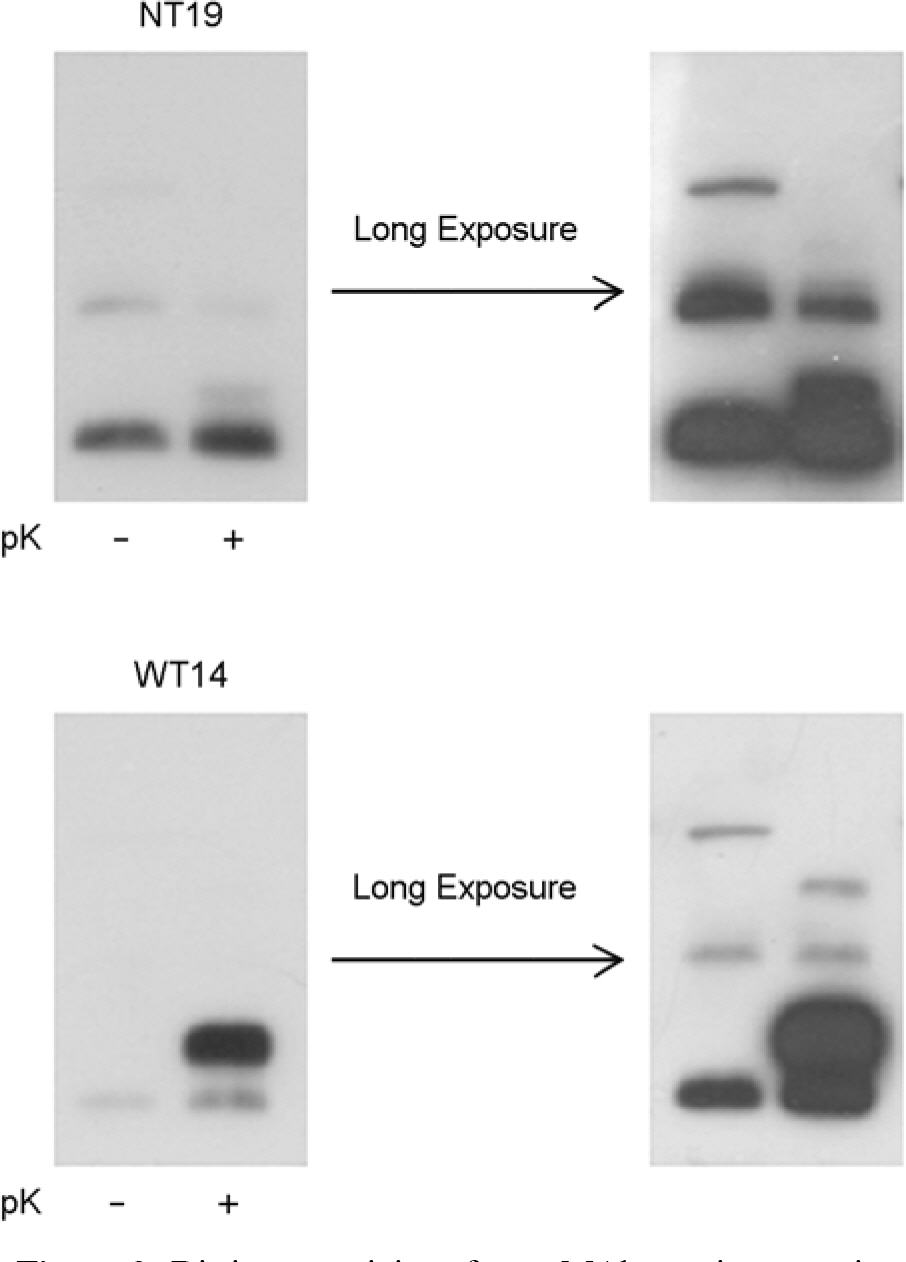
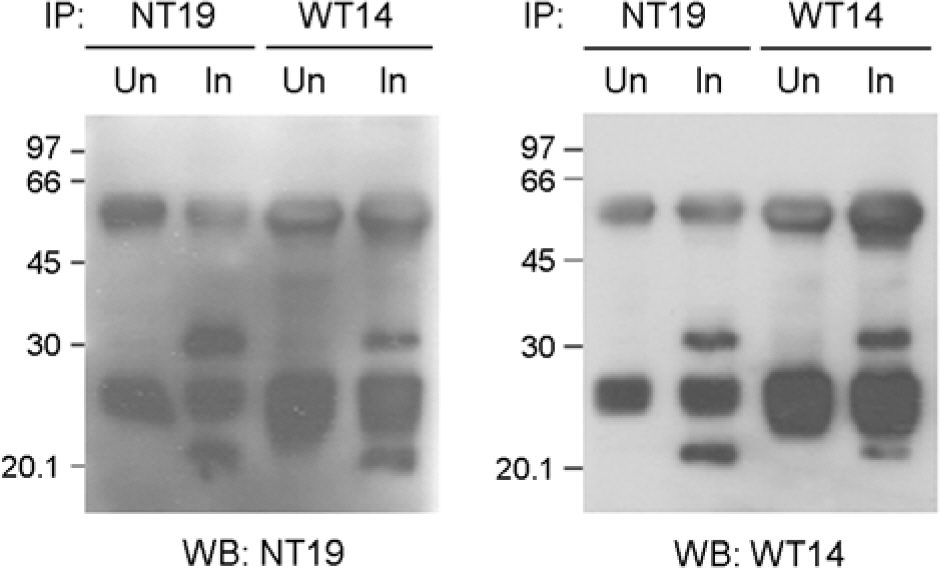
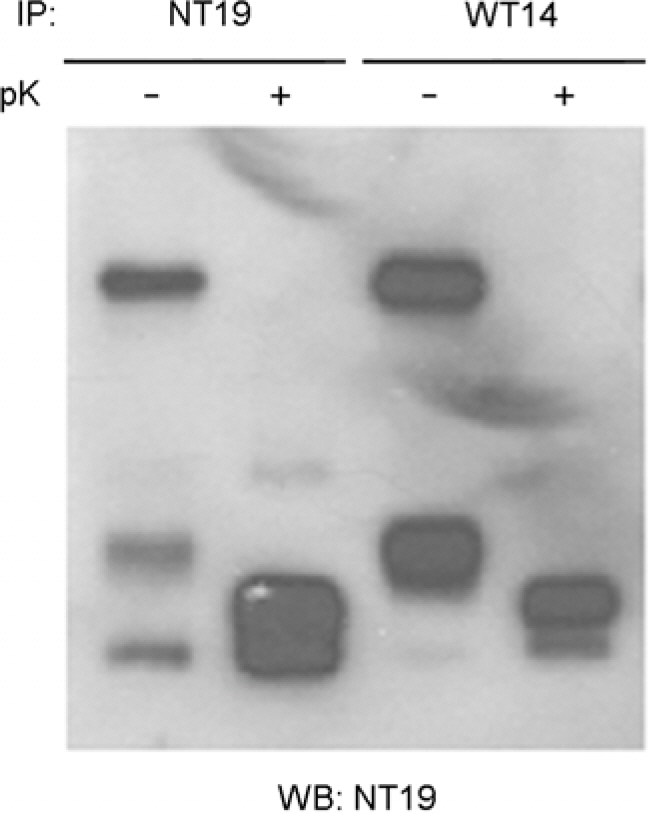
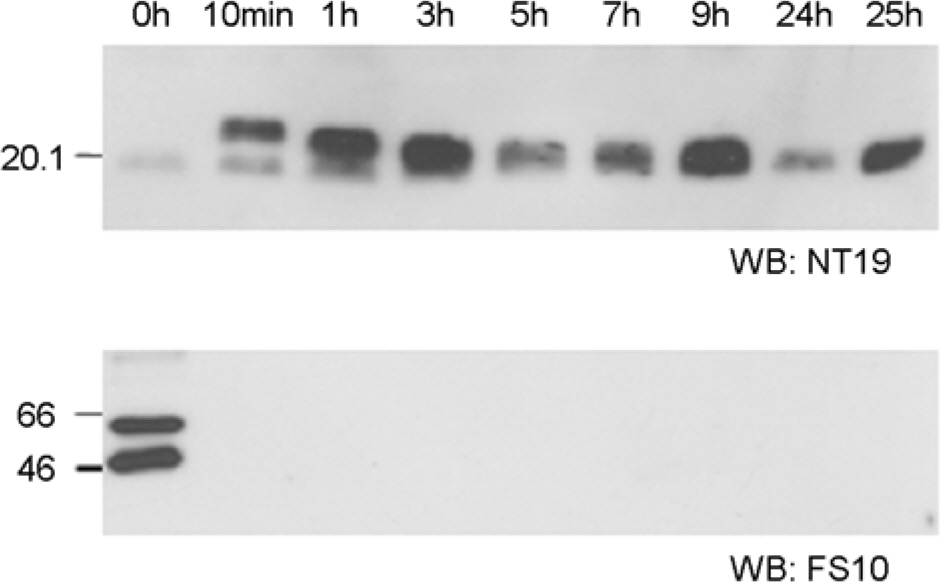
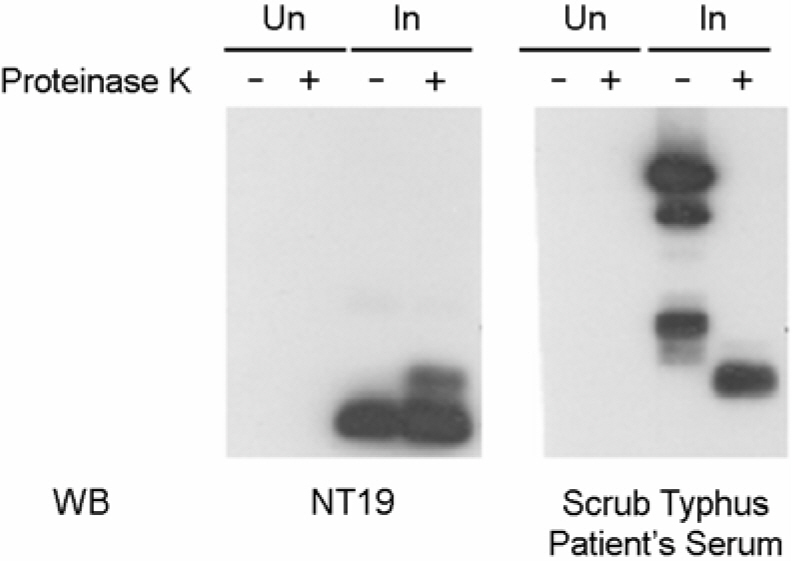
- Orientia tsutsugamushi, the causative agent of scrub typhus, is an obligate intracellular bacterium that replicates in the cytosol of host cells. Although several protein antigens have been characterized and cloned, little information exists regarding the polysaccharide antigen of this bacterium. In this study, we characterized two monoclonal antibodies, NT19 and WT14, against the proteinase K-resistant antigen of O. tsutsugamushi. Western blot analysis showed that MAb NT19 and WT14 strongly recognized two antigenic bands with molecular masses of 20 kDa and 24 kDa, which were resistant to proteinase K digestion. We suggest that the proteinase-resistant antigen might be polysaccharide. One patient serum reacted with a 24 kDa band that was similar to a band observed by WT14, suggesting the possibility of the role of this proteinase-resistant antigen as an antigenic molecule in human infection.
MeSH Terms
Figure
Reference
-
References
1). 장우현. 한국의 쯔쯔가무시병. 서흥출판사. 1994.2). Amano K, Mizushiri S, Fujii S, Fukushi K, Suto T. Immunological characterization of lipopolysaccharides from Proteus strains used in Weil-Felix test and reactivity with patient sera of tsutsugamushi diseases. Microbiol Immunol. 34:135–145. 1990.3). Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun. 55:2290–2292. 1987.4). Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 28:685–688. 1990.5). Chattopadhyay S, Jiang J, Chan TC, Manetz TS, Chao CC, Ching WM, Richards AL. Scrub typhus vaccine candidate Kp r56 induces humoral and cellular immune responses in cynomolgus monkeys. Infect Immun. 73:5039–5047. 2005.
Article6). Chattopadhyay S, Richards AL. Scrub typhus vaccines: past history and recent developments. Hum Vaccin. 3:73–80. 2007.
Article7). Cho NH, Seong SY, Choi MS, Kim IS. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect Immun. 69:1265–1272. 2001.8). Cho NH, Seong SY, Huh MS, T. Han TH, Koh YS, Choi MS, Kim IS. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 68:594–602. 2000.9). Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun. 50:603–609. 1985.10). Kaca W, Amano K, Chernyak AY, Knirel YA. Human anti-scrub typhus rickettsia and rabbit anti-Proteus antibodies recognize similar epitope in the O-polysaccharide part of Proteus mirabilis OXK lipopolysaccharide. Microbios. 103:151–161. 2000.11). Kang JS, Chang WH. Antigenic relationship among the eight prototype and new serotype strains of Orientia tsutsugamushi revealed by monoclonal antibodies. Microbiol Immunol. 43:229–234. 1999.12). Kim MK, Kang JS. Orientia tsutsugamushi suppresses the production of inflammatory cytokines induced by its own heat-stable component in murine macrophages. Microb Pathog. 31:145–150. 2001.13). Kim MK, Kee SH, Cho KA, Chung MH, Lim BU, Chang WH, Kang JS. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 43:751–757. 1999.14). Rikihisa Y, Ito S. Effect of antibody on entry of Rickettsia tsutsugamushiinto polymorphonuclear leukocyte cytoplasm. Infect Immun. 39:928–938. 1983.15). Rikihisa Y, Rota T, Lee TH, MacDonald AB, Ito S. Changes in immunoferritin labeling of Rickettsia tsutsugamushi after serial cultivation in 60Co-irradiated BHK cells. Infect Immun. 26:638–650. 1979.16). Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 3:11–21. 2001.17). Seong SY, Huh MS, Jang WJ, Park SG, Kim JG, Woo SG, Choi MS, Kim IS, Chang WH. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun. 65:1541–1545. 1997.18). Seong SY, Kim MK, Lee SM, Odgerel Z, Choi MS, Han TH, Kim IS, Kang JS, Lim BU. Neutralization epitopes on the antigenic domain II of the Orientia tsutsugamushi 56-kDa protein revealed by monoclonal antibodies. Vaccine. 19:2–9. 2000.19). Silverman DJ, Wisseman CL Jr, Waddell AD, Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 22:233–246. 1978.20). Tamura A. Invasion and intracellular growth of Rickettsia tsutsugamushi. Microbiol Sci. 5:228–232. 1988.21). Tamura A, Ohashi N, Urakami H, Takahashi K, Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 48:671–675. 1985.22). Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 182:2675–2679. 2000.
Article23). Urakami H, Tsuruhara T, Tamura A. Electron microscopic studies on intracellular multiplication of Rickettsia tsutsugamushi in L cells. Microbiol Immunol. 28:1191–1201. 1984.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentially Expressed Antigens of Orientia tsutsugamushi Revealed by Monoclonal Antibodies
- Improved Antibiotic Susceptibility Test of Orientia tsutsugamushi by Flow Cytometry Using Monoclonal Antibody
- Identification of Outer Membrane Vesicles Derived from Orientia tsutsugamushi
- Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies
- Significance of IgG and IgM antibodies in the diagnosis of scrub typhus and evaluation of rickettsia tsutsugamushi strain Boryong as a diagnostic antigen