Immune Netw.
2015 Apr;15(2):91-99. 10.4110/in.2015.15.2.91.
Anti-herpes Activity of Vinegar-processed Daphne genkwa Flos Via Enhancement of Natural Killer Cell Activity
- Affiliations
-
- 1College of Veterinary Medicine and Bio-Safety Research Institute, Specialized Campus, Chonbuk National University, Iksan 570-752, Korea. vetvirus@chonbuk.ac.kr
- 2Natural Medicine Research Center, KRIBB, Chungbuk 363-883, Korea.
- 3Department of Bioactive Materials Sciences, Graduate School, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 2168034
- DOI: http://doi.org/10.4110/in.2015.15.2.91
Abstract
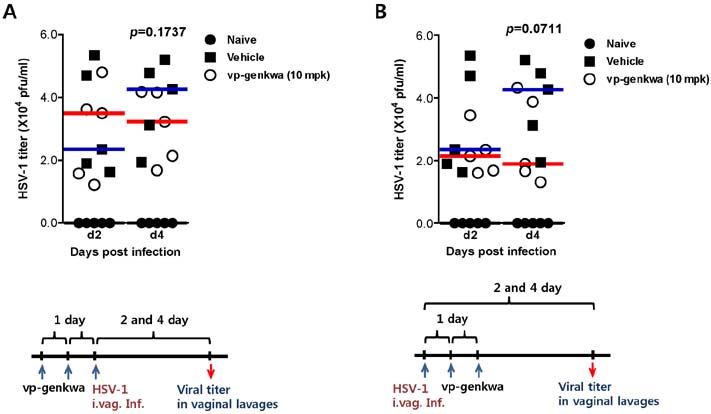
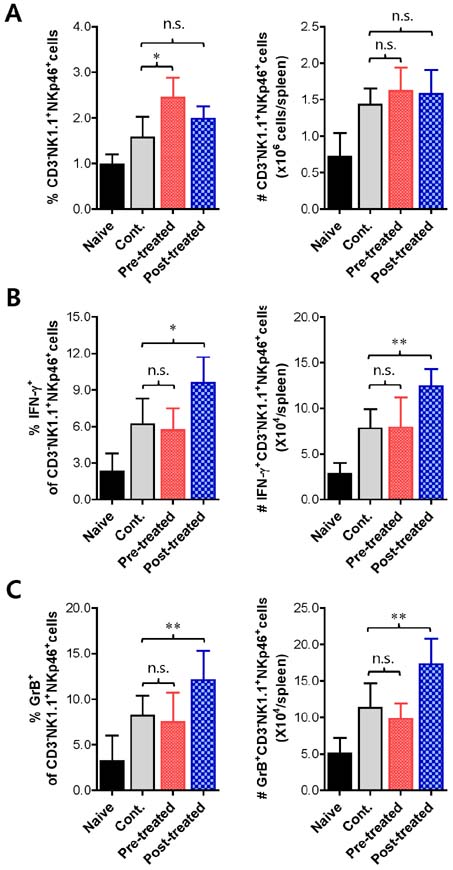
- Herpes simplex virus (HSV) is a common causative agent of genital ulceration and can lead to subsequent neurological disease in some cases. Here, using a genital infection model, we tested the efficacy of vinegar-processed flos of Daphne genkwa (vp-genkwa) to modulate vaginal inflammation caused by HSV-1 infection. Our data revealed that treatment with optimal doses of vp-genkwa after, but not before, HSV-1 infection provided enhanced resistance against HSV-1 infection, as corroborated by reduced mortality and clinical signs. Consistent with these results, treatment with vp-genkwa after HSV-1 infection reduced viral replication in the vaginal tract. Furthermore, somewhat intriguingly, treatment of vp-genkwa after HSV-1 infection increased the frequency and absolute number of CD3-NK1.1+NKp46+ natural killer (NK) cells producing interferon (IFN)-gamma and granyzme B, which indicates that vp-genkwa treatment induces the activation of NK cells. Supportively, secreted IFN-gamma was detected at an increased level in vaginal lavages of mice treated with vp-genkwa after HSV-1 infection. These results indicate that enhanced resistance to HSV-1 infection by treatment with vp-genkwa is associated with NK cell activation. Therefore, our data provide a valuable insight into the use of vp-genkwa to control clinical severity in HSV infection through NK cell activation.
MeSH Terms
Figure
Reference
-
1. Shahin V, Hafezi W, Oberleithner H, Ludwig Y, Windoffer B, Schillers H, Kuhn JE. The genome of HSV-1 translocates through the nuclear pore as a condensed rod-like structure. J Cell Sci. 2006; 119:23–30.
Article2. Daniels D, Mortlock S. Mixed HSV-1 and HSV-2 infection in a patient attending a GUM clinic. Br J Biomed Sci. 2008; 65:203–204.
Article3. Pereira VS, Moizeis RN, Fernandes TA, Araujo JM, Meissner RV, Fernandes JV. Herpes simplex virus type 1 is the main cause of genital herpes in women of Natal, Brazil. Eur J Obstet Gynecol Reprod Biol. 2012; 161:190–193.
Article4. Lee AJ, Ashkar AA. Herpes simplex virus-2 in the genital mucosa: insights into the mucosal host response and vaccine development. Curr Opin Infect Dis. 2012; 25:92–99.5. Chentoufi AA, Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol. 2012; 2012:149135.
Article6. Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013; 5:22766.
Article7. Auvert B, Ballard R, Campbell C, Carael M, Carton M, Fehler G, Gouws E, MacPhail C, Taljaard D, Van DJ, Williams B. HIV infection among youth in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behaviour. AIDS. 2001; 15:885–898.
Article8. Mugo N, Dadabhai SS, Bunnell R, Williamson J, Bennett E, Baya I, Akinyi N, Mohamed I, Kaiser R. Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis. 2011; 38:1059–1066.
Article9. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006; 20:73–83.
Article10. Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994; 70:369–380.11. Uyangaa E, Patil AM, Eo SK. Prophylactic and therapeutic modulation of innate and adaptive immunity against mucosal infection of herpes simplex virus. Immune Netw. 2014; 14:187–200.
Article12. Gill N, Rosenthal KL, Ashkar AA. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J Virol. 2005; 79:4470–4478.
Article13. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001; 82:845–853.
Article14. Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997; 229:259–268.
Article15. Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999; 258:282–294.
Article16. Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J Virol. 2001; 75:6705–6709.
Article17. Conrady CD, Jones H, Zheng M, Carr DJ. A functional type I interferon pathway drives resistance to cornea herpes simplex virus type 1 infection by recruitment of leukocytes. J Biomed Res. 2011; 25:111–119.
Article18. Conrady CD, Zheng M, Mandal NA, van RN, Carr DJ. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013; 6:45–55.
Article19. Hansen ML, Woetmann A, Krejsgaard T, Kopp KL, Sokilde R, Litman T, Straten PT, Geisler C, Wasik MA, Odum N, Eriksen KW. IFN-α primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol. 2011; 48:2087–2093.
Article20. Beuneu H, Deguine J, Bouvier I, Di Santo JP, Albert ML, Bousso P. Cutting Edge: A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J Immunol. 2011; 187:2084–2088.
Article21. Baranek T, Manh TP, Alexandre Y, Maqbool MA, Cabeza JZ, Tomasello E, Crozat K, Bessou G, Zucchini N, Robbins SH, Vivier E, Kalinke U, Ferrier P, Dalod M. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 2012; 12:571–584.
Article22. Kovalova A, Ledvina M, Saman D, Zyka D, Kubickova M, Zidek L, Sklenar V, Pompach P, Kavan D, Bily J, Vanek O, Kubinkova Z, Libigerova M, Ivanova L, Antolikova M, Mrazek H, Rozbesky D, Hofbauerova K, Kren V, Bezouska K. Synthetic N-acetyl-D-glucosamine based fully branched tetrasaccharide, a mimetic of the endogenous ligand for CD69, activates CD69+ killer lymphocytes upon dimerization via a hydrophilic flexible linker. J Med Chem. 2010; 53:4050–4065.23. Nielsen CH, Balachandran P, Christensen O, Pugh ND, Tamta H, Sufka KJ, Wu X, Walsted A, Schjorring-Thyssen M, Enevold C, Pasco DS. Enhancement of natural killer cell activity in healthy subjects by Immulina®, a Spirulina extract enriched for Braun-type lipoproteins. Planta Med. 2010; 76:1802–1808.
Article24. Jiangsu New Medical College. The Encyclopedia of Traditional Chinese medicine. 2nd ed. Shanghai Science and Technology: Shanghai;1985. p. 2573–2574.25. Zhan ZJ, Fan CQ, Ding J, Yue JM. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorg Med Chem. 2005; 13:645–655.
Article26. Li HS, Xiao ML. Research progress of the Shi-Zao-Tang to treat malignant pleural effusion and ascites. Res Integr Tradit Chin West Med. 2012; 4:93–94.27. Zheng W, Gao X, Gu Q, Chen C, Wei Z, Shi F. Antitumor activity of daphnodorins from Daphne genkwa roots. Int Immunopharmacol. 2007; 7:128–134.
Article28. Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. Phytother Res. 2006; 20:610–613.
Article29. Uyangaa E, Choi JY, Patil AM, Kim JH, Kim SB, Kim K, Ryu HW, Oh SR, Eo SK. Functional restoration of exhausted CD4+ and CD8+ T cells in chronic viral infection by vinegar-processed flos of Daphne genkwa. Comp Immunol Microbiol Infect Dis. 2015; 39:25–37.
Article30. Geng L, Sun H, Yuan Y, Liu Z, Cui Y, Bi K, Chen X. Discrimination of raw and vinegar-processed Genkwa Flos using metabolomics coupled with multivariate data analysis: a discrimination study with metabolomics coupled with PCA. Fitoterapia. 2013; 84:286–294.
Article31. Uyangaa E, Lee HK, Eo SK. Glutamine and leucine provide enhanced protective immunity against mucosal infection with herpes simplex virus type 1. Immune Netw. 2012; 12:196–206.
Article32. Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008; 8:259–268.
Article33. Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004; 172:1333–1339.
Article34. Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014; 841:15–44.
Article35. Kang HB, Ahn KS, Oh SR, Kim JW. Genkwadaphnin induces IFN-gamma via PKD1/NF-κB/STAT1 dependent pathway in NK-92 cells. PLoS One. 2014; 9:e115146.36. dib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014; 92:256–262.
Article37. Millard AL, Spirig R, Mueller NJ, Seebach JD, Rieben R. Inhibition of direct and indirect TLR-mediated activation of human NK cells by low molecular weight dextran sulfate. Mol Immunol. 2010; 47:2349–2358.
Article38. Chen S, Lin G, Lei L, You X, Wu C, Xu W, Huang M, Luo L, Wang Z, Li Y, Zhao X, Ya Fn. Hyperlipidemia modifies innate immune responses to lipopolysaccharide via the TLR-NF-κB signaling pathway. Inflammation. 2013; 36:968–976.
Article39. Langers I, Renoux V, Reschner A, Touze A, Coursaget P, Boniver J, Koch J, Delvenne P, Jacobs N. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur J Immunol. 2014; 44:3585–3595.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Natural Killer Cell Activities , T Cells and T Cell Subsets in Vitiligo
- Clinical significance of cell-mediated immunity and natural killer cell activity related with septic complication in panperitonitis patients
- The Effect of Tumor Removal and Administration of OK432 on the Splenic Natural Killer Cell Activity in the Subcutaneous Tumor Bearing Rats
- Natural killer cell activity in mice infected with Acanthamoeba culbertsoni
- Quantitation of Immune Cells (T Cells, TM, TG and B Cells) and NK Cell Activities in Patients with Herpes Zoster