Cancer Res Treat.
2014 Jul;46(3):234-242.
A Phase I Study of Oral Paclitaxel with a Novel P-Glycoprotein Inhibitor, HM30181A, in Patients with Advanced Solid Cancer
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University Ilsan Medical Center, Dogguk University College of Medicine, Goyang, Korea.
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. bangyj@snu.ac.kr
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pharmacology and Clinical Pharmacology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Konyang University Hospital, Konyang University College of Medicine, Daejeon, Korea.
- 6Department of Internal Medicine, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
- 7Department of Clinical Pharmacology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The purpose of this study is to determine the maximum tolerated dose (MTD), safety, pharmacokinetics, and recommended phase II dose of an oral drug composed of paclitaxel and HM30181A, which is an inhibitor of P-glycoprotein, in patients with advanced cancers.
MATERIALS AND METHODS
Patients with advanced solid tumors received standard therapy were given the study drug at escalating doses, using a 3+3 design. The study drug was orally administered on days 1, 8, and 15, with a 28-day cycle of administration. The dose of paclitaxel was escalated from 60 to 420 mg/m2, and the dose of HM30181A was escalated from 30-210 mg/m2.
RESULTS
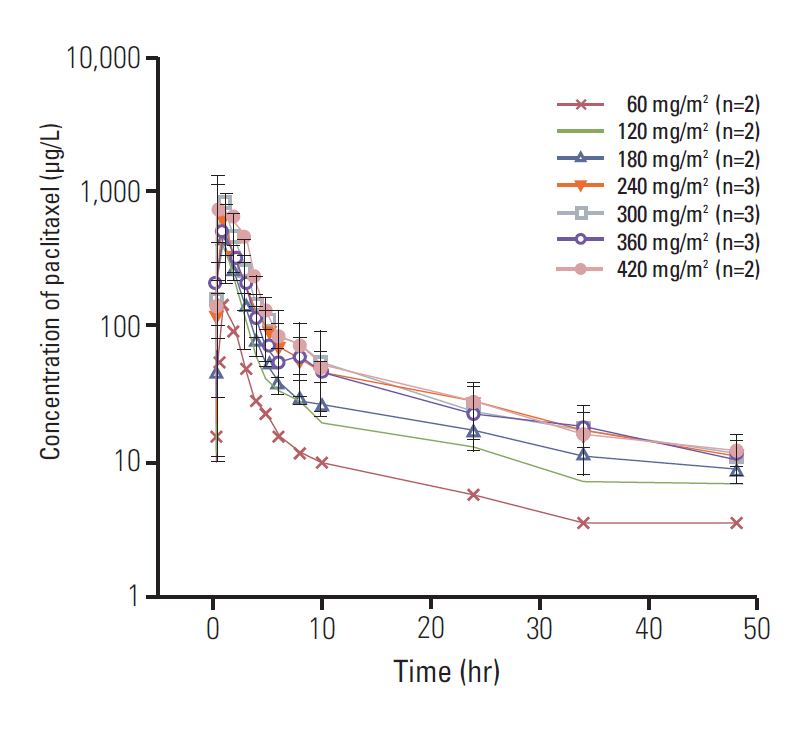
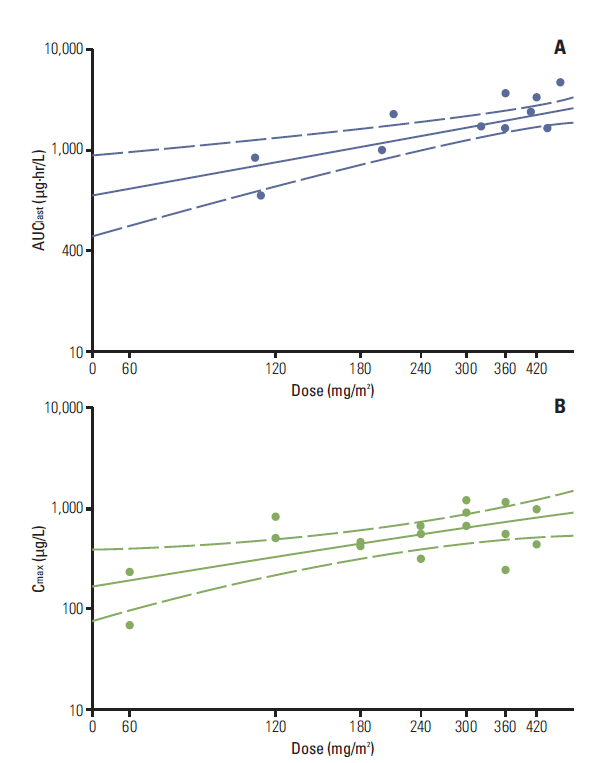
A total of twenty-four patients were enrolled. Only one patient experienced a dose-limiting toxicity-a grade 3 neutropenia that persisted for more than 2 weeks, at 240 mg/m2 of paclitaxel. MTD was not reached. The maximum plasma concentration was obtained at a dose level of 300 mg/m2 and the area under the curve of plasma concentration-time from 0 to the most recent plasma concentration measurement of paclitaxel was reached at a dose level of 420 mg/m2. The absorption of paclitaxel tends to be limited at doses that exceed 300 mg/m2. The effective plasma concentration of paclitaxel was achieved at a dose of 120 mg/m2. Responses of 23 patients were evaluated; 8 (34.8%) had stable disease and 15 (65.2%) had progressive disease.
CONCLUSION
The study drug appears to be well tolerated, and the effective plasma concentration of paclitaxel was achieved. The recommended phase II dose for oral paclitaxel is 300 mg/m2.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995; 332:1004–14.
Article2. Broker LE, Veltkamp SA, Heath EI, Kuenen BC, Gall H, Astier L, et al. A phase I safety and pharmacologic study of a twice weekly dosing regimen of the oral taxane BMS-275183. Clin Cancer Res. 2007; 13:3906–12.
Article3. Hong YS, Kim KP, Lim HS, Bae KS, Ryu MH, Lee JL, et al. A phase I study of DHP107, a mucoadhesive lipid form of oral paclitaxel, in patients with advanced solid tumors: crossover comparisons with intravenous paclitaxel. Invest New Drugs. 2013; 31:616–22.
Article4. Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997; 94:2031–5.
Article5. Malingre MM, Beijnen JH, Rosing H, Koopman FJ, van Tellingen O, Duchin K, et al. A phase I and pharmacokinetic study of bi-daily dosing of oral paclitaxel in combination with cyclosporin A. Cancer Chemother Pharmacol. 2001; 47:347–54.
Article6. Malingre MM, Beijnen JH, Rosing H, Koopman FJ, Jewell RC, Paul EM, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001; 84:42–7.
Article7. Kwak JO, Lee SH, Lee GS, Kim MS, Ahn YG, Lee JH, et al. Selective inhibition of MDR1 (ABCB1) by HM30181 increases oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol. 2010; 627:92–8.
Article8. Kim TE, Gu N, Yoon SH, Cho JY, Park KM, Shin SG, et al. Tolerability and pharmacokinetics of a new P-glycoprotein inhibitor, HM30181, in healthy Korean male volunteers: single- and multiple-dose randomized, placebo-controlled studies. Clin Ther. 2012; 34:482–94.
Article9. National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Internet]. Cancer Therapy Evaluation Program; 2006 [cited 2013 Aug 6]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.10. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.11. Paek IB, Ji HY, Kim MS, Lee GS, Lee HS. Simultaneous determination of paclitaxel and a new P-glycoprotein inhibitor HM- 30181 in rat plasma by liquid chromatography with tandem mass spectrometry. J Sep Sci. 2006; 29:628–34.12. Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000; 17:1278–83.13. Kobayashi M, Oba K, Sakamoto J, Kondo K, Nagata N, Okabayashi T, et al. Pharmacokinetic study of weekly administration dose of paclitaxel in patients with advanced or recurrent gastric cancer in Japan. Gastric Cancer. 2007; 10:52–7.
Article14. Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006; 112:457–73.
Article15. Norton L. Theoretical concepts and the emerging role of taxanes in adjuvant therapy. Oncologist. 2001; 6 Suppl 3:30–5.
Article16. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008; 26:1642–9.
Article17. Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008; 358:1663–71.
Article18. Baird RD, Tan DS, Kaye SB. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol. 2010; 7:575–82.
Article19. Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010; 7:455–65.
Article20. Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002; 62:6938–43.21. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor- free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10:3708–16.22. Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002; 8:1038–44.23. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–8.24. Darby RA, Callaghan R, McMahon RM. P-glycoprotein inhibition: the past, the present and the future. Curr Drug Metab. 2011; 12:722–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of P-Glycoprotein and Transport Mechanism of Paclitaxel in Syncytiotrophoblast Cells
- Weekly versus 3-weekly paclitaxel in combination with carboplatin in advanced ovarian cancer: which is the optimal adjuvant chemotherapy regimen?
- Phase I Clinical Trial of Paclitaxel Plus Ifosfamide for the Patients with Refractory Ovarian Cancer
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Selective cyclooxygenase inhibitors increase paclitaxel sensitivity in taxane-resistant ovarian cancer by suppressing P-glycoprotein expression