J Gynecol Oncol.
2013 Jul;24(3):273-279. 10.3802/jgo.2013.24.3.273.
Selective cyclooxygenase inhibitors increase paclitaxel sensitivity in taxane-resistant ovarian cancer by suppressing P-glycoprotein expression
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ellemedi Women's Hospital, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea. kimonc@hotmail.com
- 3Laboratory of Molecular Oncology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
- 4Clinical Development Department, CJ Cheil Jedang Pharmaceutic, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. jspark@catholic.ac.kr
- KMID: 2288535
- DOI: http://doi.org/10.3802/jgo.2013.24.3.273
Abstract
OBJECTIVE
The purpose of this study was to investigate whether selective cyclooxygenase (COX) inhibitors promote paclitaxel-induced apoptosis in taxane-resistant ovarian cancer cells by suppressing MDR1/P-glycoprotein (P-gp) expression.
METHODS
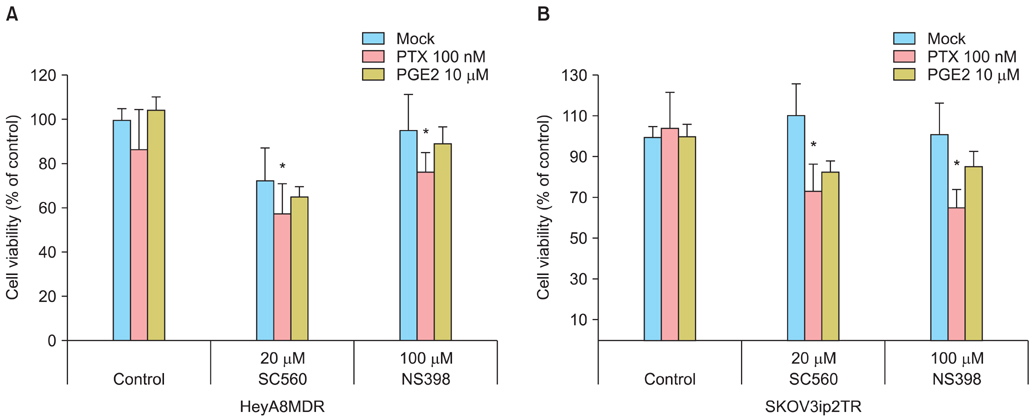
Taxane-resistant ovarian cancer cells were cultured with paclitaxel alone or combined with a selective COX inhibitors. The expression patterns of MDR1/P-gp and the ability of COX inhibitors to inhibit growth of taxane-resistant ovarian cancer cells were measured. The efficacy of prostaglandin E2 (PGE2) supplementation was measured to evaluate the mechanisms involved in suppressing MDR1 gene expression.
RESULTS
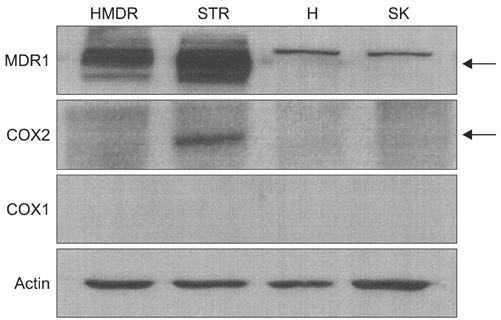
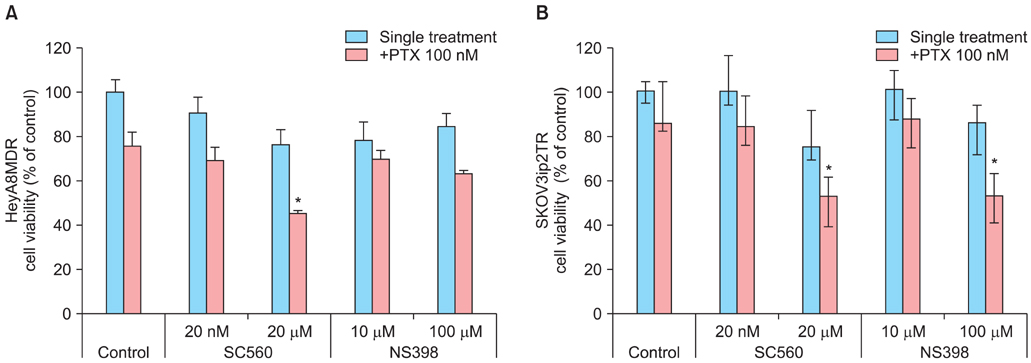
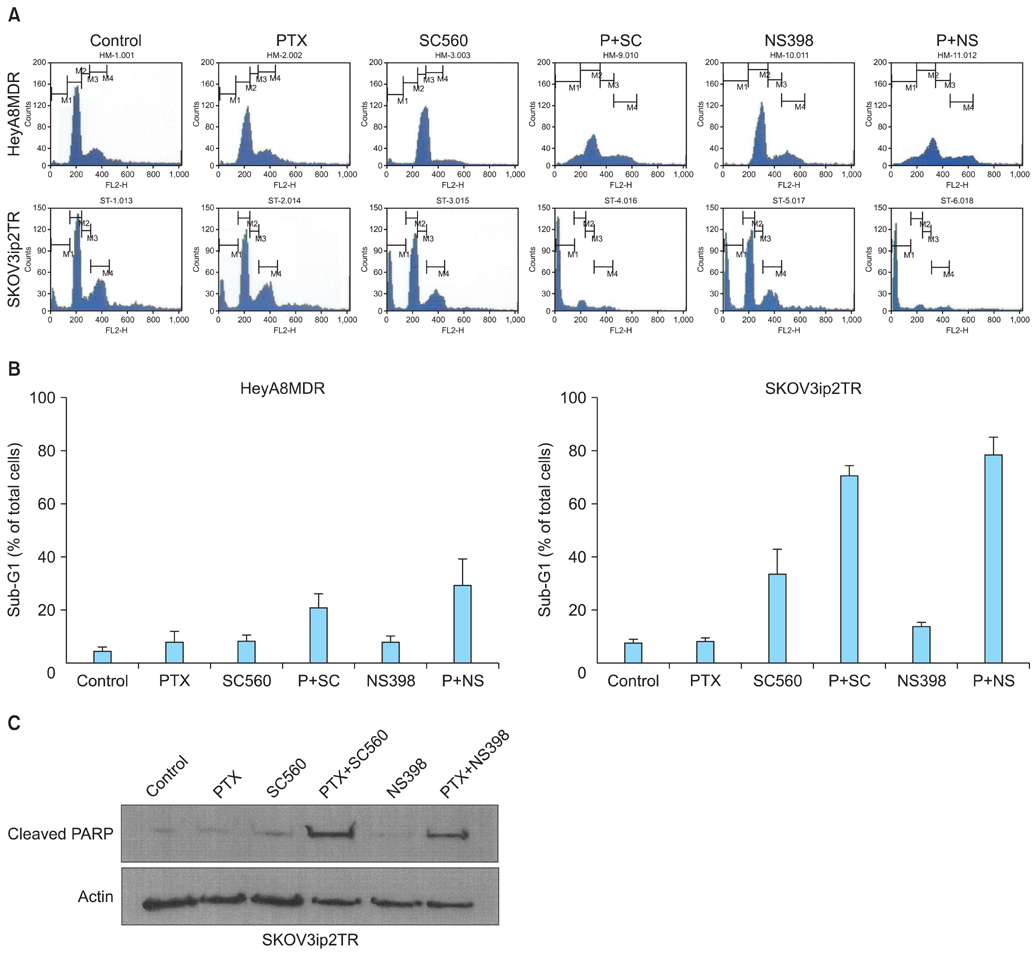
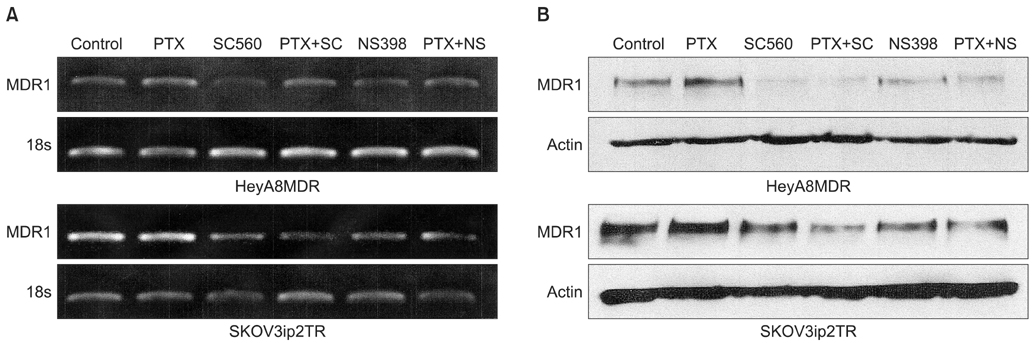
P-gp was upregulated in taxane-resistant ovarian cancer cells compared to paired paclitaxel-sensitive ovarian cancer cells. An 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that selective COX inhibitors significantly enhanced the cytotoxic effects of paclitaxel in taxane-resistant ovarian cancer cells via a prostaglandin-independent mechanism. These increased apoptotic effects were further verified by measuring an increased percentage of cells in sub-G1 stage using flow cytometry. Selective COX inhibitors suppressed MDR1 and P-gp expression. Moreover, combined treatment with paclitaxel and selective COX inhibitors increased poly (ADP-ribose) polymerase (PARP) cleavage in taxane-resistant ovarian cancer cells.
CONCLUSION
Selective COX inhibitors significantly promote paclitaxel-induced cell death in taxane-resistant ovarian cancer cells in a prostaglandin-independent manner. COX inhibitors could be potent therapeutic tools to promote paclitaxel sensitization of taxane-resistant ovarian cancers by suppressing MDR1/P-gp, which is responsible for the efflux of chemotherapeutic agents.
MeSH Terms
Figure
Reference
-
1. Yap TA, Carden CP, Kaye SB. Beyond, chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009; 9:167–181.2. Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J Gynecol Oncol. 2010; 21:75–80.3. Balch C, Huang TH, Brown R, Nephew KP. The epigenetics of ovarian cancer drug resistance and resensitization. Am J Obstet Gynecol. 2004; 191:1552–1572.4. Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cell Mol Life Sci. 2008; 65:3145–3167.5. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006; 5:219–234.6. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002; 2:48–58.7. Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008; 38:802–832.8. Denkert C, Kobel M, Pest S, Koch I, Berger S, Schwabe M, et al. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol. 2002; 160:893–903.9. Puhlmann U, Ziemann C, Ruedell G, Vorwerk H, Schaefer D, Langebrake C, et al. Impact of the cyclooxygenase system on doxorubicin-induced functional multidrug resistance 1 overexpression and doxorubicin sensitivity in acute myeloid leukemic HL-60 cells. J Pharmacol Exp Ther. 2005; 312:346–354.10. Saikawa Y, Sugiura T, Toriumi F, Kubota T, Suganuma K, Isshiki S, et al. Cyclooxygenase-2 gene induction causes CDDP resistance in colon cancer cell line, HCT-15. Anticancer Res. 2004; 24:2723–2728.11. Roy KR, Reddy GV, Maitreyi L, Agarwal S, Achari C, Vali S, et al. Celecoxib inhibits MDR1 expression through COX-2-dependent mechanism in human hepatocellular carcinoma (HepG2) cell line. Cancer Chemother Pharmacol. 2010; 65:903–911.12. Ye CG, Wu WK, Yeung JH, Li HT, Li ZJ, Wong CC, et al. Indomethacin and SC236 enhance the cytotoxicity of doxorubicin in human hepatocellular carcinoma cells via inhibiting P-glycoprotein and MRP1 expression. Cancer Lett. 2011; 304:90–96.13. Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003; 4:605–615.14. Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006; 98:736–747.15. Zrieki A, Farinotti R, Buyse M. Cyclooxygenase-2 inhibitors prevent trinitrobenzene sulfonic acid-induced P-glycoprotein up-regulation in vitro and in vivo. Eur J Pharmacol. 2010; 636:189–197.16. Wang CH, Zheng WB, Qiang O, Tang CW. Effects of non-cytotoxic drugs on the growth of multidrug-resistance human gastric carcinoma cell line. J Dig Dis. 2009; 10:91–98.17. Xia W, Zhao T, Lv J, Xu S, Shi J, Wang S, et al. Celecoxib enhanced the sensitivity of cancer cells to anticancer drugs by inhibition of the expression of P-glycoprotein through a COX-2-independent manner. J Cell Biochem. 2009; 108:181–194.18. Chuang HC, Kardosh A, Gaffney KJ, Petasis NA, Schonthal AH. COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol Cancer. 2008; 7:38.19. Li W, Zhai L, Tang Y, Cai J, Liu M, Zhang J. Antitumor properties of taxol in combination with cyclooxygenase-1 and cyclooxygenase-2 selective inhibitors on ovarian tumor growth in vivo. Oncol Res. 2012; 20:49–59.20. Li W, Tang YX, Wan L, Cai JH, Zhang J. Effects of combining Taxol and cyclooxygenase inhibitors on the angiogenesis and apoptosis in human ovarian cancer xenografts. Oncol Lett. 2013; 5:923–928.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Specific COX-2 Inhibitor, Celecoxib, on Paclitaxel-Induced Apoptosis in SK-OV-3 Epithelial Ovarian Cancer Cell Line
- A role of Cyclosporine A that suppresses multi-drug resistance (MDR) in the secondary chemotherapy drug resistant cell line of ovarian cancer
- Clusterin confers paclitaxel resistance in ovarian cancer
- Expressions of cyclooxygenase-2 and P-glycoprotein in epithelial ovarian carcinomas
- Identification of P-Glycoprotein and Transport Mechanism of Paclitaxel in Syncytiotrophoblast Cells