Cancer Res Treat.
2014 Apr;46(2):186-193.
Preclinical Efficacy Testing for Stomach and Liver Cancers
- Affiliations
-
- 1Biomolecular Function Research Branch, National Cancer Center, Goyang, Korea. hkim@ncc.re.kr
- 2Department of Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul, Korea. daeyong@snu.ac.kr
- 3National OncoVenture, Goyang, Korea.
- 4Molecular Imaging and Therapy Branch, National Cancer Center, Goyang, Korea.
Abstract
- PURPOSE
Hollow fiber assays offer an early in vivo method of anticancer drug screening. The assays have been optimized for human cancers originating from the lung, breast, colon, ovary, and brain, but not from the stomach and liver. The current study focused on optimization of hollow fiber assays for gastric and hepatocellular carcinoma cell lines.
MATERIALS AND METHODS
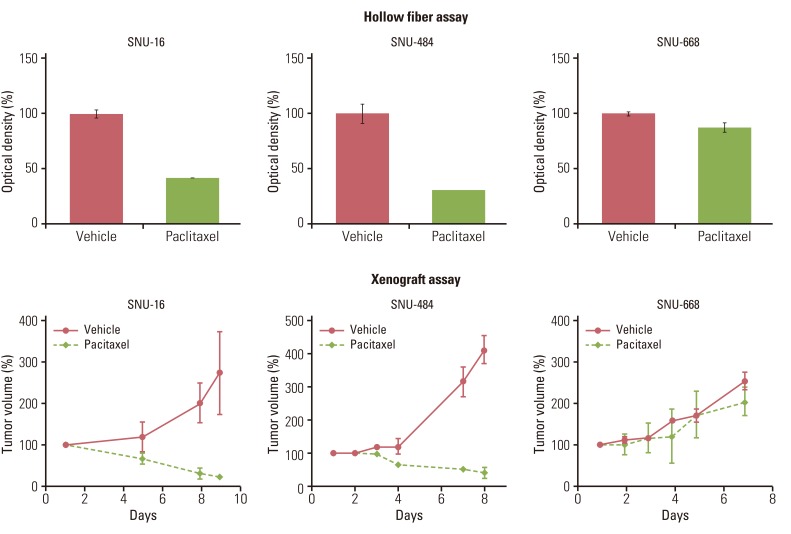
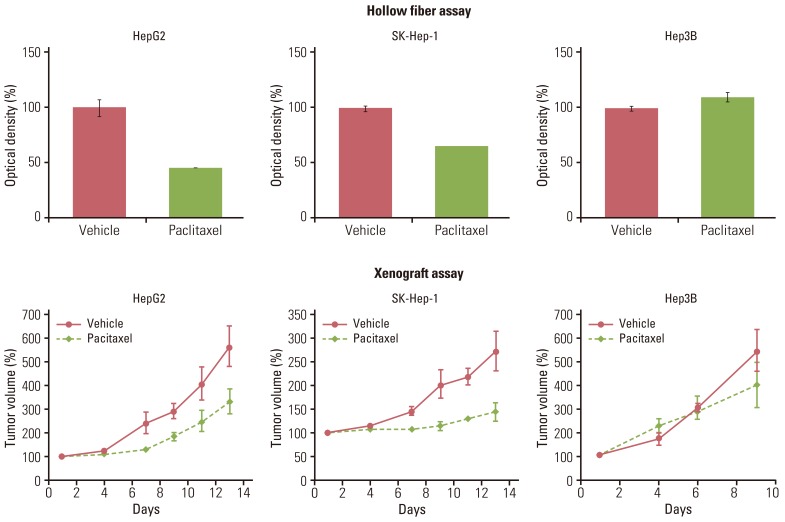
Gastric (SNU-16, SNU-484, SNU-668) and hepatocellular (HepG2, SK-Hep-1, Hep3B) carcinoma cell lines in hollow fibers were transplanted subcutaneously and intraperitoneally into mice, which were subsequently treated with a standard anticancer agent, paclitaxel. The hollow fiber activity of paclitaxel in each cell line was compared with the xenograft activity.
RESULTS
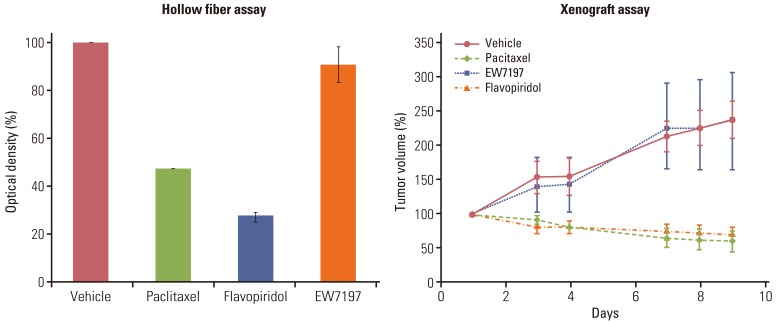
Using optimized inoculation densities and schedules, treatment with paclitaxel was effective in gastric carcinoma cell lines, SNU-16 and SNU-484, but not in SNU-668. In the hollow fiber assays, paclitaxel was effective in hepatocellular carcinoma cell lines, HepG2 and SK-Hep-1, but not in Hep3B. Consistent with the results of the hollow fiber assay, SNU-16 and SNU-484, but not SNU-668, showed tumor regression, and HepG2 and SK-Hep-1, but not Hep3B, showed effective tumor responses following treatment with paclitaxel in xenograft models. When EW7197, a novel compound, and flavopiridol were tested in SNU-16 cells under optimized conditions, the hollow fiber activity showed good correlation with the xenograft activity of each compound.
CONCLUSION
Our protocols may be useful for screening candidate small molecules that may exhibit activity against stomach and liver cancers, both of which are common in Korea.
MeSH Terms
Figure
Reference
-
1. Phillips RM, Bibby MC, Double JA. A critical appraisal of the predictive value of in vitro chemosensitivity assays. J Natl Cancer Inst. 1990; 82:1457–1468. PMID: 2202838.
Article2. Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010; 10:241–253. PMID: 20300105.
Article3. Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995; 57:131–141. PMID: 7603295.
Article4. Hall LA, Krauthauser CM, Wexler RS, Hollingshead MG, Slee AM, Kerr JS. The hollow fiber assay: continued characterization with novel approaches. Anticancer Res. 2000; 20:903–911. PMID: 10810375.5. Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001; 84:1424–1431. PMID: 11355958.
Article6. Lee KH, Rhee KH. Correlative effect between in vivo hollow fiber assay and xenografts assay in drug screening. Cancer Res Treat. 2005; 37:196–200. PMID: 19956503.
Article7. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011; 43:1–11. PMID: 21509157.
Article8. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893–1907. PMID: 20647400.
Article9. Kim SW, Kim SJ, Park SA, Kim MJ, Kim DK, Sheen YY. Anti-metastasis effect of EW-7197, a novel ALK5 inhibitor in both breast cancer cells in vitro and mouse model in vivo. Cancer Res. 2012; 72(8 Suppl 1):Abstr 1918.10. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971; 93:2325–2327. PMID: 5553076.11. Rose WC. Taxol: a review of its preclinical in vivo antitumor activity. Anticancer Drugs. 1992; 3:311–321. PMID: 1358264.12. Park JG, Frucht H, LaRocca RV, Bliss DP Jr, Kurita Y, Chen TR, et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990; 50:2773–2780. PMID: 2158397.13. Bar-Am I, Mor O, Yeger H, Shiloh Y, Avivi L. Detection of amplified DNA sequences in human tumor cell lines by fluorescence in situ hybridization. Genes Chromosomes Cancer. 1992; 4:314–320. PMID: 1377938.
Article14. Park JG, Yang HK, Kim WH, Chung JK, Kang MS, Lee JH, et al. Establishment and characterization of human gastric carcinoma cell lines. Int J Cancer. 1997; 70:443–449. PMID: 9033653.
Article15. Kim JH, Takahashi T, Chiba I, Park JG, Birrer MJ, Roh JK, et al. Occurrence of p53 gene abnormalities in gastric carcinoma tumors and cell lines. J Natl Cancer Inst. 1991; 83:938–943. PMID: 1676761.16. Heffelfinger SC, Hawkins HH, Barrish J, Taylor L, Darlington GJ. SK HEP-1: a human cell line of endothelial origin. In Vitro Cell Dev Biol. 1992; 28A:136–142. PMID: 1371504.
Article17. Sassa S, Sugita O, Galbraith RA, Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987; 143:52–57. PMID: 3030322.
Article18. Mersch-Sundermann V, Knasmuller S, Wu XJ, Darroudi F, Kassie F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology. 2004; 198:329–340. PMID: 15138059.
Article19. Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, et al. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res. 1997; 3:273–279. PMID: 9815683.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical evaluation of prototype products
- Characteristics of Synchronous Cancers in Gastric Cancer Patients
- A Case of Synchronous Primary Triple Cancers Including Stomach, Esophagus and Liver
- News Portrayal of Cancer: Content Analysis of Threat and Efficacy by Cancer Type and Comparison with Incidence and Mortality in Korea
- Estimating the Disability Weight of Major Cancers in Korea Using Delphi Method