Ann Surg Treat Res.
2014 Apr;86(4):220-225. 10.4174/astr.2014.86.4.220.
Bowel perforation associated sunitinib therapy for recurred gastric gastrointestinal stromal tumor
- Affiliations
-
- 1Department of Surgery, Chosun University Hospital, Gwangju, Korea.
- 2Department of Internal Medicine, Chosun University Hospital, Gwangju, Korea. SGPARK@chosun.ac.kr
- KMID: 2167131
- DOI: http://doi.org/10.4174/astr.2014.86.4.220
Abstract
- Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract. Several recent findings that there are activating mutations in the KIT and PDGFRA (platelet-derived growth factor receptor-alpha) genes of GISTs provide the rationale for using targeted therapies such as imatinib or sunitinib. Sunitinib, an oral multitargeted receptor tyrosine kinase inhibitor that inhibits kinases such as KIT, PDGFR (platelet-derived growth factor recepter), and VEGFR (vascular endothelial growth factor receptor), was recently approved for the treatment of imatinib-refractory GIST. Sunitinib is generally well tolerated and has an acceptable toxicity profile; an adverse event such as bowel perforation is rare. We present a patient with imatinib-refractory GIST who was successfully treated using sunitinib, but developed bowel perforation. The mechanism involved in bowel perforation associated with sunitinib is unknown. However, we presume that in our patient, the dramatic reduction in disseminated peritoneal metastases and bowel invasion of recurrent GIST during sunitinib treatment might have resulted in the bowel perforation.
MeSH Terms
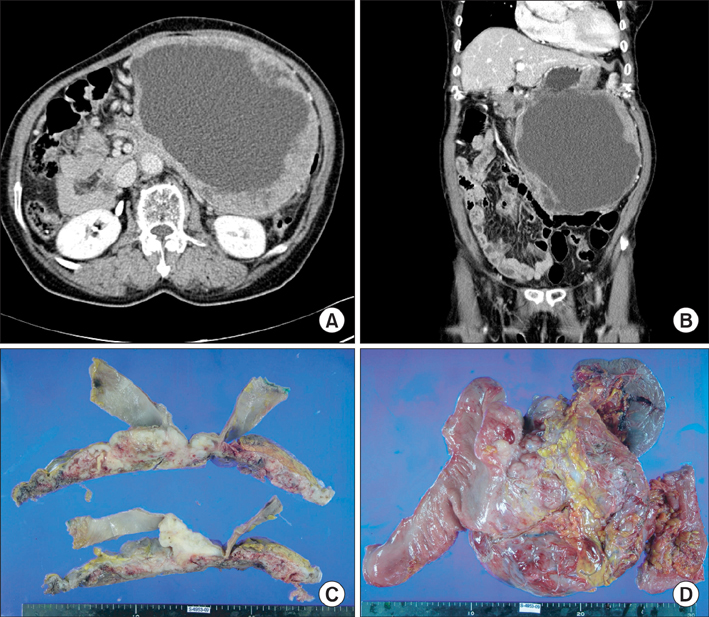
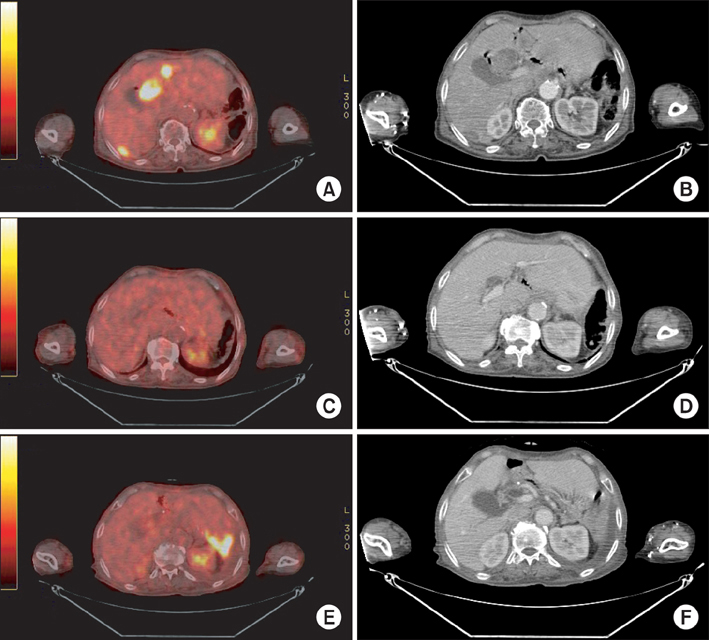
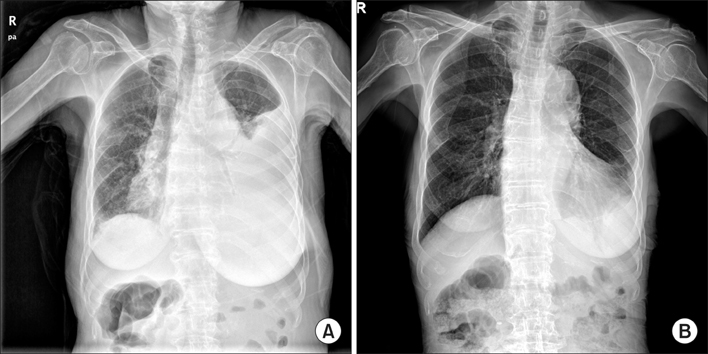
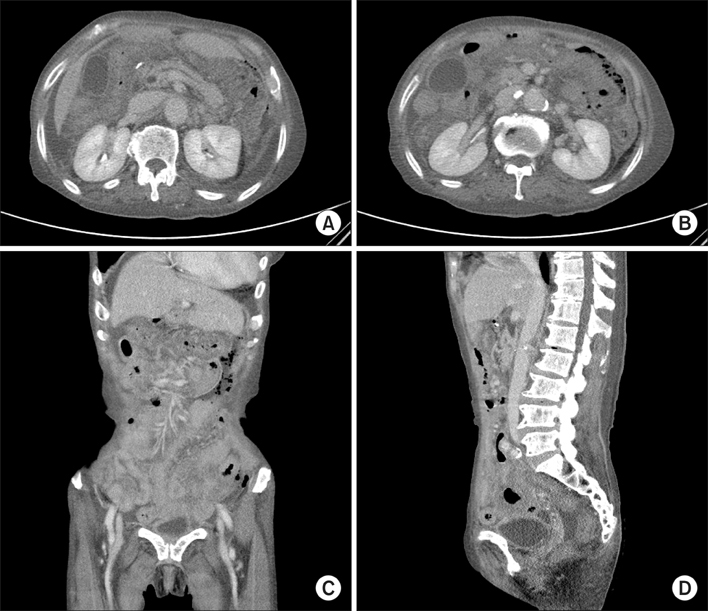
Figure
Reference
-
1. Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007; 369:1731–1741.2. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.3. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–1338.4. Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007; 12:107–113.5. Rutkowski P, Ruka W. Emergency surgery in the era of molecular treatment of solid tumours. Lancet Oncol. 2009; 10:157–163.6. Coriat R, Ropert S, Mir O, Billemont B, Chaussade S, Massault PP, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs. 2011; 29:1090–1093.7. Kim HG, Jung MR, Kang H, Cheong O, Ju JK, Park YK, et al. Discrepant bowel perforation from a primary lesion after chemotherapy of diffuse large B cell lymphoma. J Korean Surg Soc. 2009; 76:61–65.8. Hur H, Park AR, Jee SB, Jung SE, Kim W, Jeon HM. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J Gastroenterol. 2008; 14:6096–6099.9. Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007; 96:1788–1795.10. Ruka W, Rutkowski P, Szawłowski A, Nowecki Z, Debiec-Rychter M, Grzesiakowska U, et al. Surgical resection of residual disease in initially inoperable imatinib-resistant/intolerant gastrointestinal stromal tumor treated with sunitinib. Eur J Surg Oncol. 2009; 35:87–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biopsy-Proven Immune Complex Glomerulonephritis Associated with Sunitinib in a Patient with a Gastrointestinal Stromal Tumor
- A Case of Sunitinib-Induced Destructive Thyroiditis
- Sunitinib-induced hypothyroidism
- A Case of Small Bowel GIST Initially Suspected as Peritoneal Seeding of Gastric Cancer
- Gastrointestinal Stromal Tumor with Extensive Lymphatic Metastasis: A Case Report