Cancer Res Treat.
2004 Aug;36(4):235-239.
Phase II Study of Irinotecan, 5-Fluorouracil, and Leucovorin in Relapsed or Metastatic Colorectal Cancer as First-line Therapy
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Hanyang University, Seoul, Korea. ahnmj@hanyang. ac.kr
Abstract
- BACKGROUND
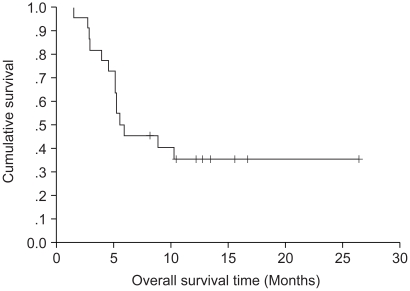
The purpose of this study was to assess the efficacy and toxicity of biweekly irinotecan plus 5-fluorouracil (FU) and leucovorin (LV) in patients with relapsed or metastatic colorectal cancer. MATERIALS AND METHODS: Between March 2002 and May 2004, 24 patients with histologically confirmed relapsed or metastatic colorectal cancer were enrolled in this study. One chemotherapy cycle consisted of irinotecan 180 mg/m2 on days 1 and 15; 5-FU 400 mg/m2 bolus IV with 600 mg/m2 by a 22 hour intravenous infusion on days 1, 2, 15 and 16; and leucovorin 20 mg/m2 on days 1, 2, 15 and 16, every 4 weeks. RESULTS: The median age of the 24 was 57.5 years (range, 38~69). Their metastatic sites included: the liver (62.5%), lung (20.8%), peritoneum (16.7%), lymph node (12.5%), ovary (8.3%) and pelvis/vagina (8.3%). Twenty- two patients were evaluable for a response. Six and 7 patients achieved partial responses and stable diseases, respectively. The overall response rate was 27.3% (95% Confidence interval; 10.3~44.5%). The median follow-up duration for surviving patients was 14.7 months (range, 1.7~26.5). Median overall survival (OS) and 1-year OS rates were 19 months and 86.3%, respectively. Median response duration and median progression free survival were 7.47 and 5.57 months, respectively. A total of 83 cycles (median 4 cycles) were administered. The main non-hematologic toxicities were nausea/vomiting (44.5%/ 18.1%) and diarrhea (8.4%). The most common hematologic toxicity was NCI grade I/II anemia (31.3%) and grade I/II neutropenia was 10.8%. There was no life-threatening toxicity. CONCLUSION: The results suggested that irinotecan, 5-FU and leucovorin combination chemotherapy in a biweekly schedule is a practical and tolerable treatment option in patients with advanced colorectal cancer.
MeSH Terms
Figure
Reference
-
1. Machover D. A comprehensive review of 5-fluorouracil and leucovorin in patients with metastatic colorectal carcinoma. Cancer. 1997; 80:1179–1187. PMID: 9317168.
Article2. Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol. 1992; 10:896–903. PMID: 1534121.3. Rothenberg ML, Meropol NJ, Poplin EA, Van Cutsem E, Wadler S. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol. 2001; 19:3801–3807. PMID: 11559717.
Article4. Kunimoto T, Nitta K, Tanaka T, Uehara N, Baba H, Takeuchi M, Yokokura T, Sawada S, Miyasaka T, Mutai M. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothecin, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987; 47:5944–5947. PMID: 3664496.5. Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, Becouarn Y, Ychou M, Marty M, Extra JM, Bonneterre J, Adenis A, Seitz JF, Ganem G, Namer M, Conroy T, Negrier S, Merrouche Y, Burki F, Mousseau M, Herait P, Mahjoubi M. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997; 15:251–260. PMID: 8996150.
Article6. Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Ghosh C, Kirschling RJ, Levitt R, Windschitl HE. Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol. 1997; 15:2910–2919. PMID: 9256135.
Article7. Rothenberg ML, Eckardt JR, Kuhn JG, Burris HA 3rd, Nelson J, Hilsenbeck SG, Rodriguez GI, Thurman AM, Smith LS, Eckhardt SG, Weiss GR, Elfring GL, Rinaldi DA, Schaaf LJ, Von Hoff DD. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol. 1996; 14:1128–1135. PMID: 8648367.
Article8. Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, Chou TC, Sun M, Pulliam S, Gonzalez C. Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol. 1996; 14:709–715. PMID: 8622015.
Article9. Andre T, Louvet C, Maindrault-Goebel F, Couteau C, Mabro M, Lotz JP, Gilles-Amar V, Krulik M, Carola E, Izrael V, de Gramont A. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. Eur J Cancer. 1999; 35:1343–1347. PMID: 10658525.10. Ducreux M, Ychou M, Seitz JF, Bonnay M, Bexon A, Armand JP, Mahjoubi M, Mery-Mignard D, Rougier P. Irinotecan combined with bolus fluorouracil, continuous infusion fluorouracil, and high-dose leucovorin every two weeks (LV5FU2 regimen): a clinical dose-finding and pharmacokinetic study in patients with pretreated metastatic colorectal cancer. J Clin Oncol. 1999; 17:2901–2908. PMID: 10561369.
Article11. Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol. 2001; 19:1501–1518. PMID: 11230497.12. Van Cutsem E, Cunningham D, Ten Bokkel Huinink WW, Punt CJ, Alexopoulos CG, Dirix L, Symann M, Blijham GH, Cholet P, Fillet G, Van Groeningen C, Vannetzel JM, Levi F, Panagos G, Unger C, Wils J, Cote C, Blanc C, Herait P, Bleiberg H. Clinical activity and benefit of irinotecan (CPT-11) in patients with colorectal cancer truly resistant to 5-fluorouracil (5-FU). Eur J Cancer. 1999; 35:54–59. PMID: 10211088.
Article13. Saltz LB, Kanowitz J, Kemeny NE, Schaaf L, Spriggs D, Staton BA, Berkery R, Steger C, Eng M, Dietz A, Locker P, Kelsen DP. Phase I clinical and pharmacokinetic study of irinotecan, fluorouracil, and leucovorin in patients with advanced solid tumors. J Clin Oncol. 1996; 14:2959–2967. PMID: 8918493.
Article14. Rothenberg ML, Cox JV, DeVore RF, Hainsworth JD, Pazdur R, Rivkin SE, Macdonald JS, Geyer CE Jr, Sandbach J, Wolf DL, Mohrland JS, Elfring GL, Miller LL, Von Hoff DD. A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer. 1999; 85:786–795. PMID: 10091755.
Article15. Vanhoefer U, Harstrick A, Kohne CH, Achterrath W, Rustum YM, Seeber S, Wilke H. Phase I study of a weekly schedule of irinotecan, high-dose leucovorin, and infusional fluorouracil as first-line chemotherapy in patients with advanced colorectal cancer. J Clin Oncol. 1999; 17:907–913. PMID: 10071283.
Article16. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000; 343:905–914. PMID: 11006366.
Article17. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000; 355:1041–1047. PMID: 10744089.
Article18. Kim JH, Kim DY, Lee SH, Park SR, Lee SY, Choi IS, Kim TY, Heo DS, Bang YJ, Kim NK. Combination chemotherapy of irinotecan combined with bolus 5-fluorouracil, continuous infusion 5-fluorouracil, and high dose leucovorin every two weeks in recurrent or metastatic colorectal cancer. Korean J Med. 2003; 64:452–458.19. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–237. PMID: 14657227.
Article20. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, Le Bail N, Haller DG. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003; 21:2059–2069. PMID: 12775730.
Article21. Kwon HC, Kim SH, Kim JS, Kim HJ. Irinotecan combined with bolus fluorouracil, continuous infusion fluorouracil, and low-dose leucovorin every two weeks in patients with oxaliplatin pretreated metastatic colorectal cancer. Cancer Res Treat. 2003; 35:135–140.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination chemotherapy of irinotecan combined with bolus 5-fluorouracil, continuous infusion 5-fluorouracil, and high dose leucovorin every two weeks in recurrent or metastatic colorectal cancer
- Phase II Study of Oxaliplatin, 5-fluorouracil, and Leucovorin in Relapsed or Metastatic Colorectal Cancer as Second Line Therapy
- Irinotecan, Continuous 5-Fluorouracil, and Low dose of Leucovorin (modified FOLFIRI) as First Line of Therapy in Recurrent or Metastatic Colorectal Cancer
- Chemotherapy for Colorecal Cancer
- 5-fluorouracil and low dose leucovorin combination chemotherapy for metastatic or recurrent colorectal cancer