Cancer Res Treat.
2006 Dec;38(4):201-205.
Phase II Study of Oxaliplatin, 5-fluorouracil, and Leucovorin in Relapsed or Metastatic Colorectal Cancer as Second Line Therapy
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Hanyang University, Korea. jhcmd@hanyang.ac.kr
- 2Department of Internal Medicine, College of Medicine, Sungkyunkwan University, Seoul, Korea.
- 3Department of Internal Medicine, Gangneung Asan Hospital, Gangneung, Korea.
Abstract
- PURPOSE
The purpose of the study was to assess the efficacy and safety of biweekly oxaliplatin in combination with leucovorin (LV)-modulated bolus plus infusion of 5-fluorouracil (5-FU) in patients with relapsed or metastatic colorectal cancer (CRC) as a second line therapy.
MATERIALS AND METHODS
Between November 2002 and October 2005, 26 patients with histologically confirmed relapsed or metastatic CRC were enrolled. All patients were previously treated with irinotecan-based combination chemotherapy. The chemotherapy regimen consisted of oxaliplatin 85 mg/m2 on day 1; LV 200 mg/m2 on days 1 and 2; and 5-FU 400 mg/m2 bolus IV with 600 mg/m2 with a 22-hour infusion on days 1 and 2 every 2 weeks.
RESULTS
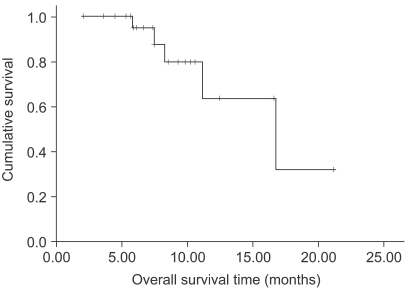
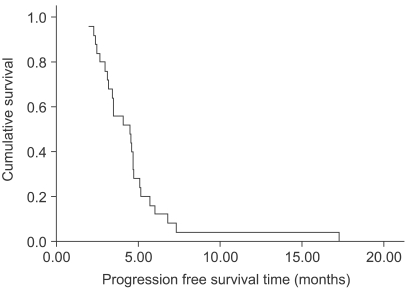
The median age of the 26 patients was 50.5 years (range, 31~72). Their metastatic sites included: the liver (42.3%), peritoneum (26.9%), lung (23.1%) and ovary (7.7%). Twenty five patients were evaluated for their response. Four patients achieved partial responses and 15 patients had stable disease. The overall response rate was 16% (95% confidence interval; 1.7~30.3%). The median follow-up duration for the surviving patients was 7.4 months (range, 2.08~21.2). Median overall survival (OS) and 1-year OS rates were 16.7 months and 63.9%, respectively. The most common hematological toxicities were: NCI grade I/II leucopenia (49.3%), grade I/II neutropenia (41%) and grade I/II anemia (65.2%). The main non-hematological toxicities were: grade I/II peripheral neuropathy (16.1% and 21.5%, respectively) and nausea/ vomiting (23.6%/18.5%). There was no life-threatening toxicity.
CONCLUSION
The oxaliplatin, 5-FU and LV combination chemotherapy, scheduled as a biweekly protocol, was effective and well tolerated in the treatment of relapsed or metastatic colorectal cancer patients as second line chemotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Machover D. A comprehensive review of 5-fluorouracil and leucovorin in patients with metastatic colorectal carcinoma. Cancer. 1997; 80:1179–1187. PMID: 9317168.
Article2. Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993; 306:752–755. PMID: 7683942.
Article3. Kunimoto T, Nitta K, Tanaka T, Uehara N, Baba H, Takeuchi M, et al. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothec in, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987; 47:5944–5947. PMID: 3664496.4. Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997; 15:251–260. PMID: 8996150.
Article5. Ducreux M, Ychou M, Seitz JF, Bonnay M, Bexon A, Armand JP, et al. Irinotecan combined with bolus fluorouracil, continuous infusion fluorouracil, and high-dose leucovorin every two weeks (LV5FU2 regimen): a clinical dose-finding and pharmacokinetic study in patients with pretreated metastatic colorectal cancer. J Clin Oncol. 1999; 17:2901–2908. PMID: 10561369.
Article6. Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998; 25(2):Suppl 5. 4–12. PMID: 9609103.7. Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996; 52:1855–1865. PMID: 8951344.
Article8. Becouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol. 1998; 16:2739–2744. PMID: 9704726.9. Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996; 7:95–98. PMID: 9081400.
Article10. Kouroussis C, Souglakos J, Mavroudis D, Papadouris S, Kakolyris S, Agelaki S, et al. Oxaliplatin with high-dose leucovorin and infusional 5-fluorouracil in irinotecan-pretreated patients with advanced colorectal cancer (ACC). Am J Clin Oncol. 2002; 25:627–631. PMID: 12478014.
Article11. Extra JM, Espie M, Calvo F, Ferme C, Miqnot L, Marty M. Phase I study of oxaliplatin in patients with advanced cancer. Cancer Chemother Pharmacol. 1990; 25:299–303. PMID: 2295116.
Article12. Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, de Braud F, et al. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: a phase II multicentric study. Ann Oncol. 1998; 9:105–108. PMID: 9541691.13. Kouroussis C, Souqlakos J, Kakolyris S, Mavroudis D, Malamos N, Kalbakis K, et al. Oxaliplatin in combination with infusional 5-fluorouracil and leucovorin every 2 weeks as first-line treatment in patients with advanced colorectal cancer: a phase II study. Oncology. 2001; 61:36–41. PMID: 11474246.
Article14. Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999; 17:3560–3568. PMID: 10550155.15. Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000; 18:136–147. PMID: 10623704.
Article16. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947. PMID: 10944126.
Article17. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–237. PMID: 14657227.
Article18. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003; 21:2059–2069. PMID: 12775730.
Article19. Maindrault-Goebel F, de Gramont A, Louvet C, Andre T, Carola E, Mabro M, et al. High-dose intensity oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX 7). Eur J Cancer. 2001; 37:1000–1005. PMID: 11334725.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leucovorin-induced Hypersensitivity Reaction in a Patient with Metastatic Colorectal Cancer Treated with Cetuximab Plus FOLFOX Chemotherapy: A Case Report
- The Efficacy of a Modified Chronomodulated Infusion of Oxaliplatin, 5-Fluorouracil and Leucovorin in Advanced Colorectal Cancer (Preliminary Data)
- Comparison between Responder and Non- responder of Oxaliplatin Chemotherapy for Metastatic Colorectal Cancer
- A Phase II Study of Oxaliplatin, 5-Fluorouracil, and Leucovorin in 5- Fluorouracil-Pretreated Metastatic Colorectal Cancer
- Combination of oxaliplatin, fluorouracil, and leucovorin in the treatment of fluoropyrimidine-pretreated patients with metastatic colorectal cancer