Cancer Res Treat.
2006 Dec;38(4):206-213.
Gemcitabine Single or Combination Chemotherapy in Post Anthracycline and Taxane Salvage Treatment of Metastatic Breast Cancer: Retrospective Analysis of 124 Patients
- Affiliations
-
- 1Division of Oncology, Department of Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sbkim3@amc.seoul.kr
- 2Departments of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Departments of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Departments of Diagnostic Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To evaluate the efficacy of gemcitabine- based chemotherapy, particularly in patients with anthracycline- and taxane-pretreated 2(nd)-line or greater metastatic breast cancer, and to compare gemcitabine monotherapy (G) with two gemcitabine-based doublets, gemcitabine/ vinorelbine (GV) and gemcitabine/capecitabine (GX).
MATERIALS AND METHODS
Of 124 consecutive patients who progressed after anthracycline- and taxane-containing chemotherapy, 58 received G alone, 38 received GV, and 28 received GX; their outcomes were analyzed retrospectively.
RESULTS
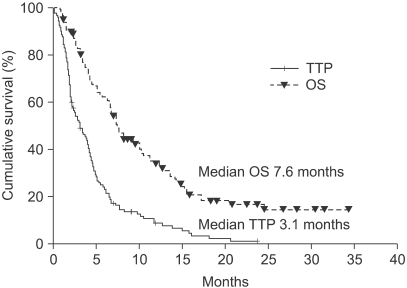
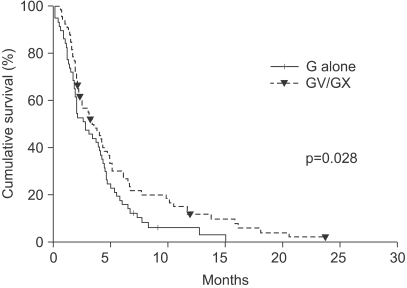
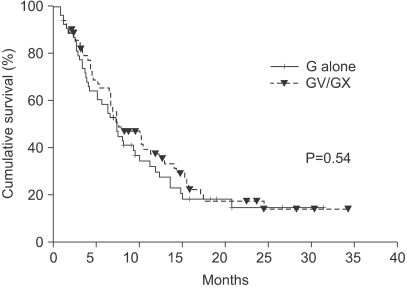
The median number of prior metastatic chemotherapy regimens was 2 (range 0~4). Visceral metastases were observed in 65 patients (51.4%). The overall response rate was 19.3% (21 partial responses). After a median follow-up period of 21.4 months, the overall survival was 7.6 months (95% CI: 5.5~9.6 months) and the median time to progression was 3.1 months (95% CI: 2.0~4.2 months). Compared with monotherapy (G), combination therapy with vinorelbine or capecitabine (GV/ GX) was associated with a significantly higher response rate (8.2% vs. 28.3%, p=0.008) and a significantly longer median time to progression (2.8 vs. 3.5 months; p=0.028), but overall survival did not differ between the groups (7.4 vs. 8.2 months, respectively; p=0.54). Most of the adverse treatment-related events were mild to moderate in intensity. The most common adverse event was hematologic toxicity. Multivariate analysis showed that poor performance status and a short disease-free interval were independent prognostic factors for impaired overall survival.
CONCLUSIONS
The combination of gemcitabine with vinorelbine or capecitabine was an active and well-tolerated treatment option for taxane- and anthracycline-pretreated 2(nd)-line or greater metastatic breast cancer patients, and gemcitabine-based doublets were more beneficial than gemcitabine monotherapy in alleviating symptoms for these patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Cardoso F, Di LA, Lohrisch C, Bernard C, Ferreira F, Piccart MJ. Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann Oncol. 2002; 13:197–207. PMID: 11885995.
Article2. Plunkett W, Huang P, Searcy CE, Gandhi V. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996; 23(5):suppl 10. 3–15. PMID: 8893876.3. Casper ES, Green MR, Kelsen DP, Heelan RT, Brown TD, Flombaum CD, et al. Phase II trial of gemcitabine (2,2'-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs. 1994; 12:29–34. PMID: 7960602.4. Cormier Y, Eisenhauer E, Muldal A, Gregg R, Ayoub J, Goss G, et al. Gemcitabine is an active new agent in previously untreated extensive small cell lung cancer (SCLC). A study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 1994; 5:283–285. PMID: 8186176.5. Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1994; 12:1821–1826. PMID: 8083706.
Article6. O'Rourke TJ, Brown TD, Havlin K, Kuhn JG, Craig JB, Burris HA, et al. Phase I clinical trial of gemcitabine given as an intravenous bolus on 5 consecutive days. Eur J Cancer. 1994; 30A:417–418. PMID: 8204374.7. Possinger K, Kaufmann M, Coleman R, Stuart NS, Helsing M, Ohnmacht U, et al. Phase II study of gemcitabine as first-line chemotherapy in patients with advanced or metastatic breast cancer. Anticancer Drugs. 1999; 10:155–162. PMID: 10211545.
Article8. Spielmann M, Llombart-Cussac A, Kalla S, Espie M, Namer M, Ferrero JM, et al. Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology. 2001; 60:303–307. PMID: 11408796.
Article9. Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase II trial. Oncology. 2002; 62:2–8. PMID: 11810037.
Article10. Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005; 90:215–221. PMID: 15830134.
Article11. Park JY, Kim C, Sohn JH, Kim YT, Rha SY, Jang WI, et al. A phase II study of gemcitabine monotherapy in breast cancer refractory to anthracycline and taxane. Cancer Res Treat. 2002; 34:274–279.12. Dickson RB, Pestell RG, Lippman ME. DeVita VT, Hellman S, Rosenberg SA, editors. Cancer of the breast. Cancer: principles & practice of oncology. 2005. 7th ed. Philadelphia P.A.: Lippincott Williams and Wilkins;p. 1399–1487.13. Heinemann V, Stemmler HJ, Wohlrab A, Bosse D, Losem C, Kahlert S, et al. High efficacy of gemcitabine and cisplatin in patients with predominantly anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2006; 57:640–646. PMID: 16163537.
Article14. Zielinski C, Beslija S, Mrsic-Krmpotic Z, Welnicka-Jaskiewicz M, Wiltschke C, Kahan Z, et al. Gemcitabine, epirubicin, and paclitaxel versus fluorouracil, epirubicin, and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: a Central European Cooperative Oncology Group International, multicenter, prospective, randomized phase III trial. J Clin Oncol. 2005; 23:1401–1408. PMID: 15735116.
Article15. Stathopoulos GP, Rigatos SK, Pergantas N, Tsavdarides D, Athanasiadis I, Malamos NA, et al. Phase II trial of biweekly administration of vinorelbine and gemcitabine in pretreated advanced breast cancer. J Clin Oncol. 2002; 20:37–41. PMID: 11773151.
Article16. Nicolaides C, Dimopoulos MA, Samantas E, Bafaloukos D, Kalofonos C, Fountzilas G, et al. Gemcitabine and vinorelbine as second-line treatment in patients with metastatic breast cancer progressing after first-line taxane-based chemotherapy: a phase II study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2000; 11:873–875. PMID: 10997817.
Article17. Andres R, Mayordomo JI, Lara R, Lastra R, Ortega E, Polo E, et al. Gemcitabine/capecitabine in patients with metastatic breast cancer pretreated with anthracyclines and taxanes. Clin Breast Cancer. 2005; 6:158–162. PMID: 16001994.
Article18. Sledge GW, Neuberg D, Bernardo P, Ingle JN, Martino S, Rowinsky EK, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol. 2003; 21:588–592. PMID: 12586793.
Article19. Alba E, Martin M, Ramos M, Adrover E, Balil A, Jara C, et al. Spanish Breast Cancer Research Group. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol. 2004; 22:2587–2593. PMID: 15226326.
Article20. O'Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002; 20:2812–2823. PMID: 12065558.21. Munoz M, Martin M, Ruiz A, Balil A, Garcia-Mata J, Calvo L, et al. Phase III study of gemcitabine plus vinorelbine (VG) versus vinorelbine (V) in patients with metastatic breast cancer (MBC) previously treated with anthracyclines (A) and taxanes (T). Final results of GEICAM 2000-04 study. Proc Am Soc Clin Oncol. 2006; 24:18S. (abstr 575).22. Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, et al. Multicentre phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004; 40:536–542. PMID: 14962720.
Article23. Mavroudis D, Malamos N, Alexopoulos A, Kourousis C, Agelaki S, Sarra E, et al. Greek Breast Cancer Cooperative Group. Salvage chemotherapy in anthracycline-pretreated metastatic breast cancer patients with docetaxel and gemcitabine: a multicenter phase II trial. Ann Oncol. 1999; 10:211–215. PMID: 10093691.
Article24. Alexopoulos A, Tryfonopoulos D, Karamouzis MV, Gerasimidis G, Karydas I, Kandilis K, et al. Evidence for in vivo synergism between docetaxel and gemcitabine in patients with metastatic breast cancer. Ann Oncol. 2004; 15:95–99. PMID: 14679126.
Article25. Hennessy BT, Gauthier AM, Michaud LB, Hortobagyi G, Valero V. Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at M. D. Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol. 2005; 16:1289–1296. PMID: 15890665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gemcitabine and Vinorelbine Combination Chemotherapy in Anthracycline- and Taxane-pretreated Advanced Breast Cancer
- Treatment with Cisplatin and Etoposide Chemotherapy in Patient with Metastatic Breast Cancer
- Phase II Study of Gemcitabine plus Cisplatin in Patients with Anthracycline- and Taxane- Pretreated Metastatic Breast Cancer
- Maintenance chemotherapy after 6 cycles of platinum-doublet regimen in anthracycline-and taxane-pretreated metastatic breast cancer
- A Phase II Study of Gemcitabine Monotherapy in Breast Cancer Patients Refractory to Anthracycline and Taxane