J Korean Med Sci.
2015 Aug;30(8):1189-1196. 10.3346/jkms.2015.30.8.1189.
Mechanical Antiallodynic Effect of Intrathecal Nefopam in a Rat Neuropathic Pain Model
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, Pusan National University School of Medicine; Research Institute for Convergence of biomedical science and technology Pusan National University Yangsan Hospital, Yangsan, Korea. byeongj@pusan.ac.kr
- 2Division of Meridian and Structural Medicine, Pusan National University School of Korean Medicine, Yangsan, Korea.
- KMID: 2164516
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1189
Abstract
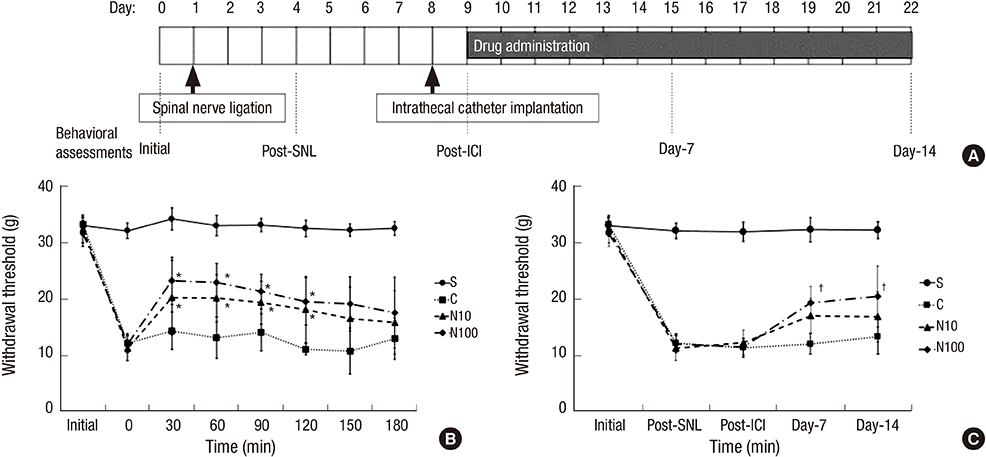
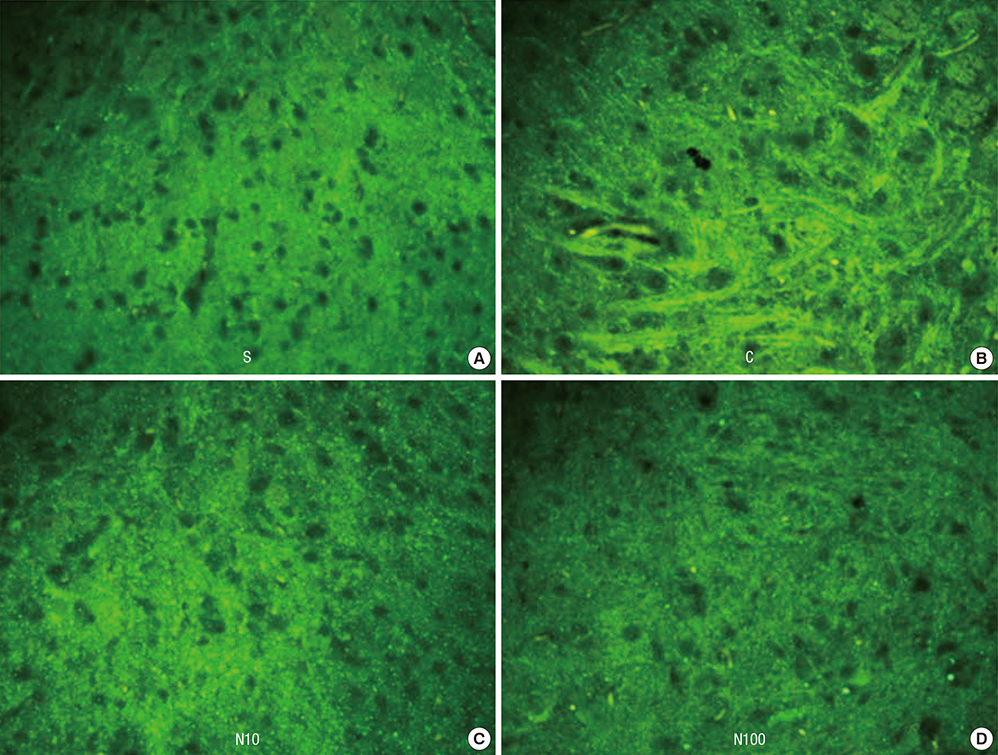
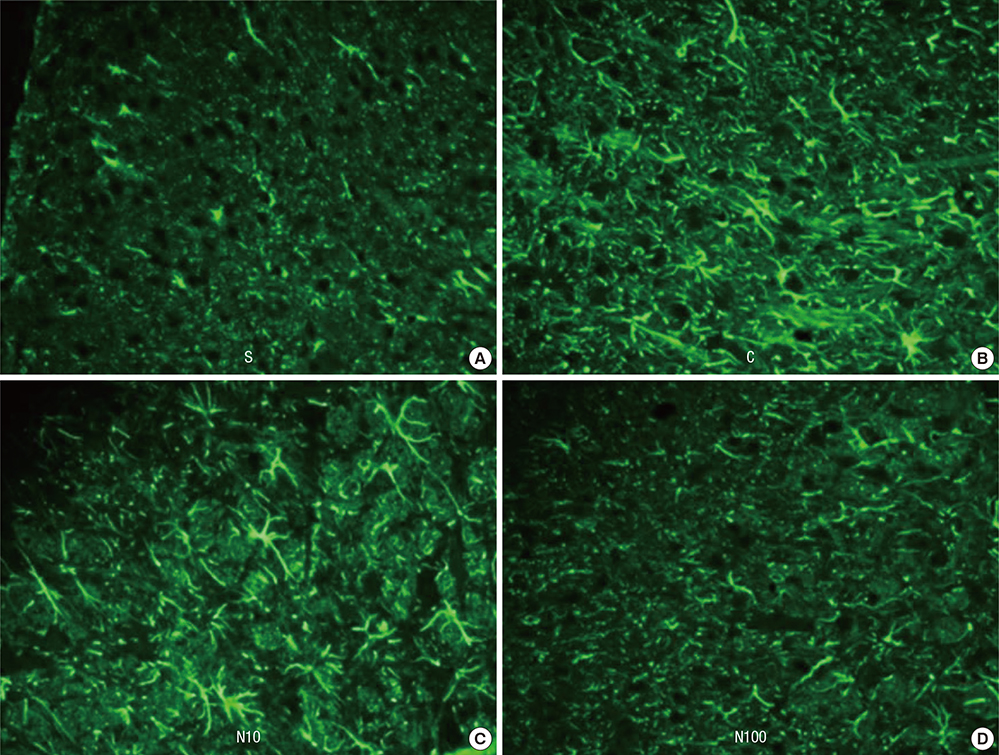
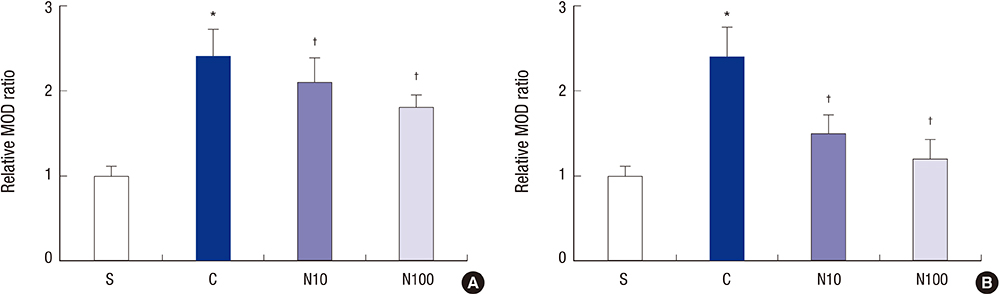
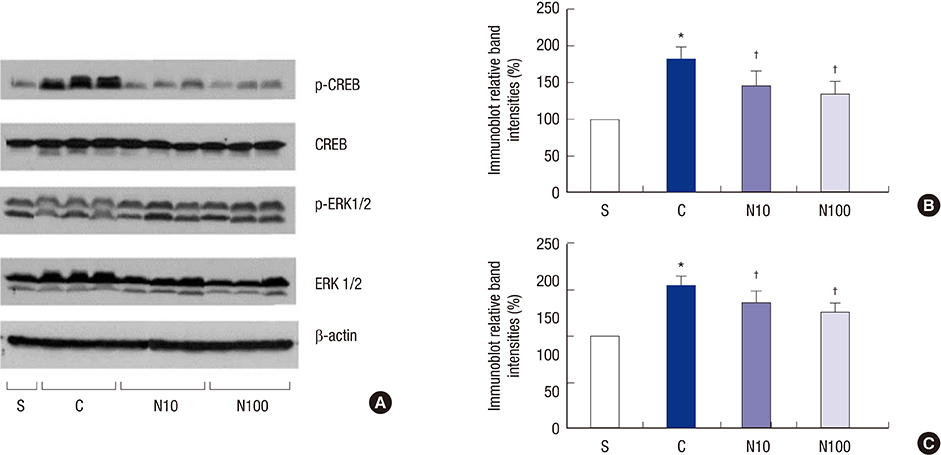
- Nefopam has a pharmacologic profile distinct from that of opioids or other anti-inflammatory drugs. Several recent studies demonstrate that nefopam has a mechanism of action similar to those of anti-depressants and anticonvulsants for treating neuropathic pain. The present study investigates the mechanical antiallodynic effect of nefopam using immunohistochemical study and western blot analysis in a rat neuropathic pain model. Twenty-eight male Sprague-Dawley rats were subjected to left fifth lumbar (L5) spinal nerve ligation and intrathecal catheter implantation, procedures which were not performed on the 7 male Sprague-Dawley rats in the sham surgery group (group S). Nefopam, either 10 or 100 microg/kg (group N10 or N100, respectively), and normal saline (group C) were intrathecally administered into the catheter every day for 14 days. The mechanical allodynic threshold of intrathecal nefopam was measured using a dynamic plantar aesthesiometer. Immunohistochemistry targeting cluster of differentiation molecule 11b (CD11b) and glial fibrillary acidic protein (GFAP) was performed on the harvested spinal cord at the level of L5. Extracellular signal-regulated kinase 1/2 (ERK 1/2) and cyclic adenosine monophosphate response element binding protein (CREB) were measured using western blot analysis. The N10 and N100 groups showed improved mechanical allodynic threshold, reduced CD11b and GFAP expression, and attenuated ERK 1/2 and CREB in the affected L5 spinal cord. In conclusion, intrathecal nefopam reduced mechanical allodynia in a rat neuropathic pain model. Its mechanical antiallodynic effect is associated with inhibition of glial activation and suppression of the transcription factors' mitogen-activated protein kinases in the spinal cord.
MeSH Terms
-
Analgesics, Non-Narcotic/administration & dosage
Animals
Dose-Response Relationship, Drug
Hyperalgesia/*drug therapy/etiology/*physiopathology
Injections, Spinal
Male
Nefopam/*administration & dosage
Neuralgia/complications/*drug therapy/*physiopathology
Pain Measurement/drug effects
Pain Perception/*drug effects
Rats
Rats, Sprague-Dawley
Treatment Outcome
Analgesics, Non-Narcotic
Nefopam
Figure
Cited by 2 articles
-
Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
Jeong Il Choi
Korean J Pain. 2016;29(2):71-72. doi: 10.3344/kjp.2016.29.2.71.Earlier treatment improves the chances of complete relief from postherpetic neuralgia
Dong Hee Kang, Su Young Kim, Hyuck Goo Kim, Jung Hyun Park, Tae Kyun Kim, Kyung Hoon Kim
Korean J Pain. 2017;30(3):214-219. doi: 10.3344/kjp.2017.30.3.214.
Reference
-
1. Lee HJ, Shin SW, Jang HJ. The combined antiallodynic effect of gabapentin and milnacipran in a rat neuropathic pain model. Korean J Pain. 2007; 20:8–14.2. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999; 83:389–400.3. Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, Fontana DJ. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 1997; 324:153–160.4. Idänpään-Heikkilä JJ, Guilbaud G. Pharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviour. Pain. 1999; 79:281–290.5. Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004; 311:576–584.6. Guirimand F, Dupont X, Bouhassira D, Brasseur L, Chauvin M. Nefopam strongly depresses the nociceptive flexion (R(III)) reflex in humans. Pain. 1999; 80:399–404.7. Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003; 20:593–601.8. Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, Marnett L, Roth BL, Shoichet BK. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci U S A. 2012; 109:11178–11183.9. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363.10. Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005; 114:149–159.11. Song XS, Cao JL, Xu YB, He JH, Zhang LC, Zeng YM. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin. 2005; 26:789–798.12. Ma W, Quirion R. Increased phosphorylation of cyclic AMP response element-binding protein (CREB) in the superficial dorsal horn neurons following partial sciatic nerve ligation. Pain. 2001; 93:295–301.13. Kang G, Choi KY, Lee MS, Ahn YJ, Kang SS, Cheong IY, Chun W, Kim SS. Minocycline attenuates the development of allodynia: an immunohistochemical study on CD11b, GFAP and c-Fos in the spinal dorsal horn in SD rat. Korean J Pathol. 2004; 38:311–318.14. Saghaei E, Moini Zanjani T, Sabetkasaei M, Naseri K. Enhancement of antinociception by co-administrations of nefopam, morphine, and nimesulide in a rat model of neuropathic pain. Korean J Pain. 2012; 25:7–15.15. Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology. 1993; 32:995–999.16. Rosland JH, Hole K. The effect of nefopam and its enantiomers on the uptake of 5-hydroxytryptamine, noradrenaline and dopamine in crude rat brain synaptosomal preparations. J Pharm Pharmacol. 1990; 42:437–438.17. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255.18. Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002; 100:1–6.19. Millan MJ. Descending control of pain. Prog Neurobiol. 2002; 66:355–474.20. Fasmer OB, Berge OG, Jørgensen HA, Hole K. Antinociceptive effects of (+/-)-, (+)- and (-)-nefopam in mice. J Pharm Pharmacol. 1987; 39:508–511.21. Piercey MF, Schroeder LA. Spinal and supraspinal sites for morphine and nefopam analgesia in the mouse. Eur J Pharmacol. 1981; 74:135–140.22. Cho SY, Park AR, Yoon MH, Lee HG, Kim WM, Choi JI. Antinociceptive effect of intrathecal nefopam and interaction with morphine in formalin-induced pain of rats. Korean J Pain. 2013; 26:14–20.23. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005; 6:521–532.24. Mollace V, Colasanti M, Muscoli C, Lauro GM, Iannone M, Rotiroti D, Nistico G. The effect of nitric oxide on cytokine-induced release of PGE2 by human cultured astroglial cells. Br J Pharmacol. 1998; 124:742–746.25. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996; 19:312–318.26. Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003; 104:655–664.27. Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005; 115:71–83.28. Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006; 199:397–407.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanical Antiallodynic Effect of intrathecal Morphine and R-Phenylisopropyl-Adenosine in Rats with Spinal Nerve Ligation
- The Combined Antiallodynic Effect of Gabapentin and Milnacipran in a Rat Neuropathic Pain Model
- Comparison of Antiallodynic Effects between Intrathecal Cholinesterase Inhibitors and NMDA Antagonists on Two Neuropathic Pain Rat Models
- Comparison of the Antiallodynic Effects of Intrathecal Lidocaine and MK-801 on Mechanical Allodynia in Two Neuropathic Pain Rat Models
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain