J Korean Med Sci.
2015 Aug;30(8):1183-1188. 10.3346/jkms.2015.30.8.1183.
Dicer Is Down-regulated and Correlated with Drosha in Idiopathic Sudden Sensorineural Hearing Loss
- Affiliations
-
- 1Department of Immunology, School of Medicine, Keimyung University, Daegu, Korea.
- 2Department of Anatomy, School of Medicine, Keimyung University, Daegu, Korea.
- 3Department of Otorhinolaryngology, School of Medicine, Keimyung University, Daegu, Korea. entnamsi@dsmc.or.kr
- KMID: 2164515
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1183
Abstract
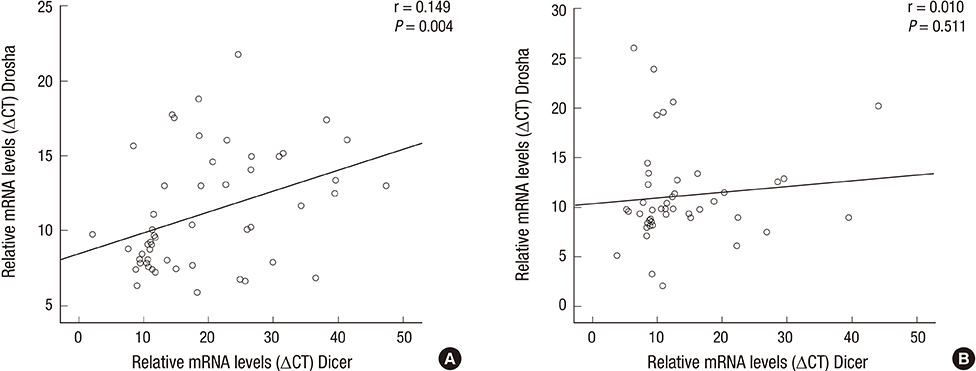
- Previously, we reported the expression levels of specific microRNA machinery components, DGCR8 and AGO2, and their clinical association in patients with idiopathic sudden hearing loss (SSNHL). In the present study, we investigated the other important components of microRNA machinery and their association with clinical parameters in SSNHL patients. Fifty-seven patients diagnosed with SSNHL and fifty healthy volunteers were included in this study. We evaluated mRNA expression levels of Dicer and Drosha in whole blood of patients with SSNHL and the control group, using RT & real-time PCR analysis. The Dicer mRNA expression level was down-regulated in patients with SSNHL. However, the Drosha mRNA expression level was not significantly altered in patients with SSNHL. Neither the Dicer nor Drosha mRNA expression level was not associated with any clinical parameters, including age, sex, duration of initial treatment from onset (days), initial Pure tone average, Siegel's criteria, WBC, and Erythrocyte sedimentation rate. However, mRNA expression levels of Dicer and Drosha were positively correlated to each other in patients with SSNHL. In this study, we demonstrated for the first time that the Dicer mRNA expression level was down-regulated in patients with SSNHL, suggesting its important role in pathobiology of SSNHL development.
MeSH Terms
-
Acute Disease
Adult
Biomarkers
DEAD-box RNA Helicases/*blood
Down-Regulation
Female
Gene Expression Regulation
Hearing Loss, Sensorineural/*blood
Hearing Loss, Sudden/*blood
Humans
Male
MicroRNAs/*metabolism
Middle Aged
Ribonuclease III/*blood/*metabolism
Statistics as Topic
Biomarkers
DEAD-box RNA Helicases
MicroRNAs
Ribonuclease III
Figure
Reference
-
1. Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010; 120:1011–1021.2. Fusconi M, Chistolini A, Angelosanto N, Pignoloni P, Tombolini M, De Virgilio A, Pagliarella M, de Vincentiis M. Role of genetic and acquired prothrombotic risk factors in genesis of sudden sensorineural hearing loss. Audiol Neurootol. 2011; 16:185–190.3. Nam SI, Yu GI, Kim HJ, Park KO, Chung JH, Ha E, Shin DH. A polymorphism at -1607 2G in the matrix metalloproteinase-1 (MMP-1) increased risk of sudden deafness in Korean population but not at -519A/G in MMP-1. Laryngoscope. 2011; 121:171–175.4. Nam SI, Ha E, Jung KH, Baik HH, Yoon SH, Park HJ, Choe BK, Chung JH, Seo JC, Lee MY, et al. IL4 receptor polymorphism is associated with increased risk of sudden deafness in Korean population. Life Sci. 2006; 78:664–667.5. Uchida Y, Sugiura S, Ando F, Shimokata H, Nakashima T. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with sudden sensorineural hearing loss. Laryngoscope. 2010; 120:791–795.6. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297.7. Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013; 329:125–136.8. Li PY, He FC, Zhou GQ. Association of human microRNA related genetic variations with cancer. Yi Chuan. 2011; 33:870–878.9. Schmittgen TD. Regulation of microRNA processing in development, differentiation and cancer. J Cell Mol Med. 2008; 12:1811–1819.10. Papachristou DJ, Korpetinou A, Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, Scopa CD, Kalofonos HP. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch. 2011; 459:431–440.11. Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004; 432:235–240.12. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003; 425:415–419.13. Dalmay T. MicroRNAs and cancer. J Intern Med. 2008; 263:366–375.14. Tijsterman M, Plasterk RH. Dicers at RISC; the mechanism of RNAi. Cell. 2004; 117:1–3.15. Han SY, Kim S, Shin DH, Cho JH, Nam SI. The Expression of AGO2 and DGCR8 in Idiopathic Sudden Sensorineural Hearing Loss. Clin Exp Otorhinolaryngol. 2014; 7:269–274.16. Inchley CS, Sonerud T, Fjaerli HO, Nakstad B. Reduced Dicer expression in the cord blood of infants admitted with severe respiratory syncytial virus disease. BMC Infect Dis. 2011; 11:59.17. Tao J, Wu H, Lin Q, Wei W, Lu XH, Cantle JP, Ao Y, Olsen RW, Yang XW, Mody I, et al. Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration. J Neurosci. 2011; 31:8306–8319.18. Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, Torriani M, Yki-Järvinen H, Grinspoon SK, Cypess AM, et al. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014; 124:3339–3351.19. Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008; 359:2641–2650.20. Sand M, Skrygan M, Georgas D, Arenz C, Gambichler T, Sand D, Altmeyer P, Bechara FG. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2012; 51:916–922.21. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3:1101–1108.22. Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, et al. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004; 270:488–498.23. Cheng JC, Moore TB, Sakamoto KM. RNA interference and human disease. Mol Genet Metab. 2003; 80:121–128.24. Lin CC, Chang YM, Pan CT, Chen CC, Ling L, Tsao KC, Yang RB, Li WH. Functional evolution of cardiac microRNAs in heart development and functions. Mol Biol Evol. 2014; 31:2722–2734.25. Lee Y, Han J, Yeom KH, Jin H, Kim VN. Drosha in primary microRNA processing. Cold Spring Harb Symp Quant Biol. 2006; 71:51–57.26. Devasthanam AS, Tomasi TB. Dicer in immune cell development and function. Immunol Invest. 2014; 43:182–195.27. Torres A, Torres K, Paszkowski T, Jodłowska-Jędrych B, Radomański T, Książek A, Maciejewski R. Major regulators of microRNAs biogenesis Dicer and Drosha are down-regulated in endometrial cancer. Tumour Biol. 2011; 32:769–776.28. Sand M, Gambichler T, Skrygan M, Sand D, Scola N, Altmeyer P, Bechara FG. Expression levels of the microRNA processing enzymes Drosha and dicer in epithelial skin cancer. Cancer Invest. 2010; 28:649–653.29. Cheng C, Fu X, Alves P, Gerstein M. mRNA expression profiles show differential regulatory effects of microRNAs between estrogen receptor-positive and estrogen receptor-negative breast cancer. Genome Biol. 2009; 10:R90.30. Passon N, Gerometta A, Puppin C, Lavarone E, Puglisi F, Tell G, Di Loreto C, Damante G. Expression of Dicer and Drosha in triple-negative breast cancer. J Clin Pathol. 2012; 65:320–326.31. Mockenhaupt S, Schürmann N, Grimm D. When cellular networks run out of control: global dysregulation of the RNAi machinery in human pathology and therapy. Prog Mol Biol Transl Sci. 2011; 102:165–242.32. Byl FM Jr. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. 1984; 94:647–661.33. Jaffe BF. Clinical studies in sudden deafness. Adv Otorhinolaryngol. 1973; 20:221–228.34. Murai K, Tsuiki T, Kusano H, Shishido K. Clinical study of audiograms in the initial stage and fixed stage of sudden deafness. Acta Otolaryngol Suppl. 1994; 514:17–20.35. Saeki N, Kitahara M. Assessment of prognosis in sudden deafness. Acta Otolaryngol Suppl. 1994; 510:56–61.36. Enache R, Sarafoleanu C. Prognostic factors in sudden hearing loss. J Med Life. 2008; 1:343–347.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Hyperbaric Oxygen in Treatment of Idiopathic Sudden Sensorineural Hearing Loss
- Stellate Ganglion Block in Pediatric Patient with Idiopathic Sudden Sensorineural Hearing Loss : A case report
- Hyperbaric Oxygen Therapy for Sudden Sensorineural Hearing Loss after Failure from Oral and Intratympanic Corticosteroid
- The Characteristics and the Changes of Tinnitus according to the Recovery of Hearing Loss in the Patients with Sudden Hearing Loss
- Medulloblastoma Manifesting as Sudden Sensorineural Hearing Loss