Yonsei Med J.
2015 Sep;56(5):1322-1327. 10.3349/ymj.2015.56.5.1322.
Ivy Sign on Fluid-Attenuated Inversion Recovery Images in Moyamoya Disease: Correlation with Clinical Severity and Old Brain Lesions
- Affiliations
-
- 1Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kylee@yuhs.ac
- 2Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Neurosurgery, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Severance Institute for Vascular and Metabolic Research, Seoul, Korea.
- KMID: 2163624
- DOI: http://doi.org/10.3349/ymj.2015.56.5.1322
Abstract
- PURPOSE
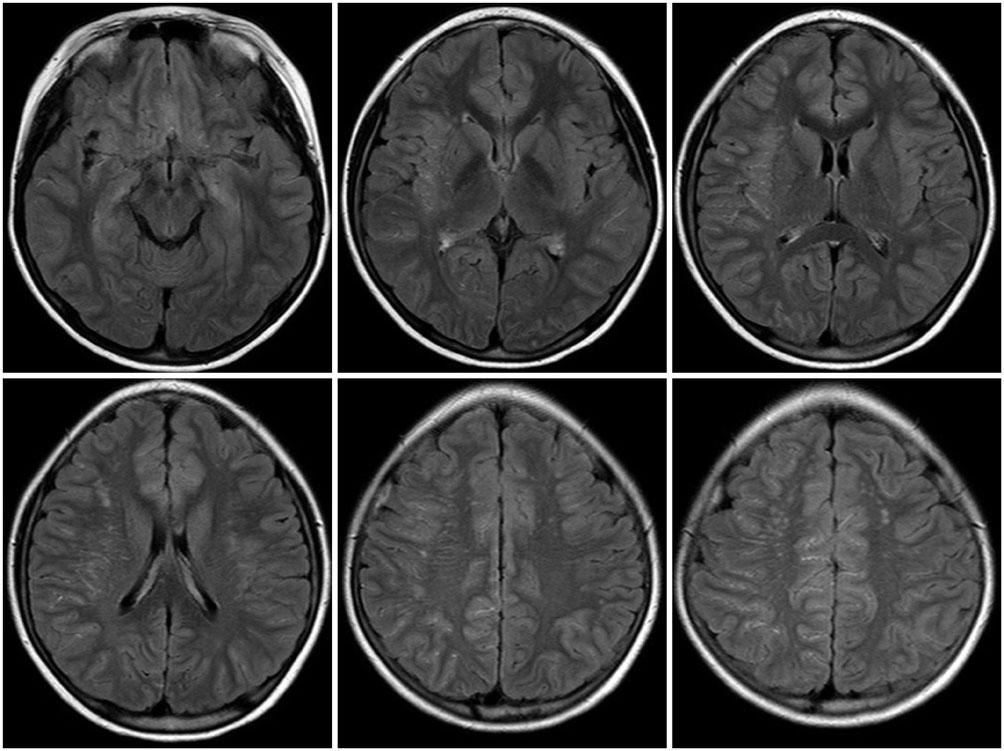
Leptomeningeal collateral, in moyamoya disease (MMD), appears as an ivy sign on fluid-attenuated inversion-recovery (FLAIR) images. There has been little investigation into the relationship between presentation of ivy signs and old brain lesions. We aimed to evaluate clinical significance of ivy signs and whether they correlate with old brain lesions and the severity of clinical symptoms in patients with MMD.
MATERIALS AND METHODS
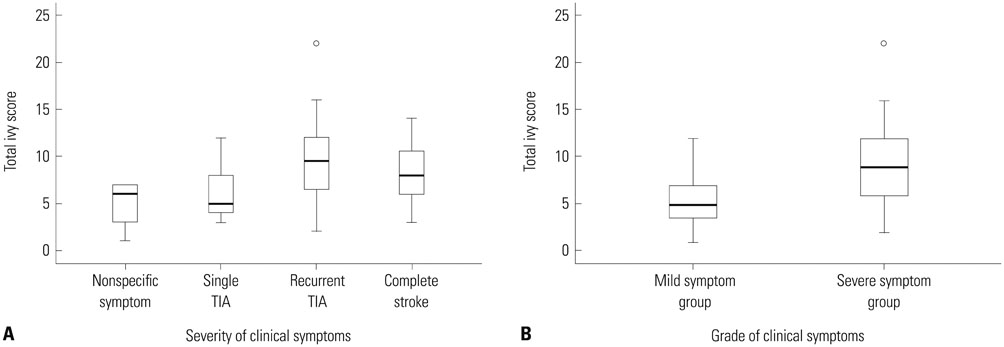
FLAIR images of 83 patients were reviewed. Each cerebral hemisphere was divided into 4 regions and each region was scored based on the prominence of the ivy sign. Total ivy score (TIS) was defined as the sum of the scores from the eight regions and dominant hemispheric ivy sign (DHI) was determined by comparing the ivy scores from each hemisphere. According to the degree of ischemic symptoms, patients were classified into four subgroups: 1) nonspecific symptoms without motor weakness, 2) single transient ischemic attack (TIA), 3) recurrent TIA, or 4) complete stroke.
RESULTS
TIS was significantly different as follows: 4.86+/-2.55 in patients with nonspecific symptoms, 5.89+/-3.10 in patients with single TIA, 9.60+/-3.98 in patients with recurrent TIA and 8.37+/-3.39 in patients with complete stroke (p=0.003). TIS associated with old lesions was significantly higher than those not associated with old lesions (9.35+/-4.22 vs. 7.49+/-3.37, p=0.032). We found a significant correlation between DHI and motor symptoms (p=0.001).
CONCLUSION
Because TIS has a strong tendency with severity of ischemic motor symptom and the presence of old lesions, the ivy sign may be useful in predicting severity of disease progression.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Brain/metabolism/*pathology
Cerebral Arteries/*pathology
Child
Child, Preschool
Collateral Circulation
Disease Progression
Female
Humans
Magnetic Resonance Imaging/*methods
Male
Meninges/*pathology
Middle Aged
Moyamoya Disease/complications/*pathology
Severity of Illness Index
Stroke
Young Adult
Figure
Cited by 1 articles
-
Acute Ischemic Stroke in Moyamoya Syndrome Associated with Thyrotoxicosis
Donggook Kang, Gi-Hun Seong, Jong Seok Bae, Ju-Hun Lee, Hong-Ki Song, Yerim Kim
J Neurocrit Care. 2018;11(2):129-133. doi: 10.18700/jnc.180046.
Reference
-
1. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 20:288–299.2. Takeuchi K, Shimizu K. Hypogenesis of bilateral internal carotid artery. Shinkei. 1957; 9:37–43.3. Maeda M, Tsuchida C. "Ivy sign" on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR Am J Neuroradiol. 1999; 20:1836–1838.4. Fujiwara H, Momoshima S, Kuribayashi S. Leptomeningeal high signal intensity (ivy sign) on fluid-attenuated inversion-recovery (FLAIR) MR images in moyamoya disease. Eur J Radiol. 2005; 55:224–230.
Article5. Kawashima M, Noguchi T, Takase Y, Ootsuka T, Kido N, Matsushima T. Unilateral hemispheric proliferation of ivy sign on fluid-attenuated inversion recovery images in moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR Am J Neuroradiol. 2009; 30:1709–1716.
Article6. Mori N, Mugikura S, Higano S, Kaneta T, Fujimura M, Umetsu A, et al. The leptomeningeal "ivy sign" on fluid-attenuated inversion recovery MR imaging in Moyamoya disease: a sign of decreased cerebral vascular reserve? AJNR Am J Neuroradiol. 2009; 30:930–935.
Article7. Ishikawa T, Kuroda S, Nakayama N, Terae S, Kudou K, Iwasaki Y. Prevalence of asymptomatic microbleeds in patients with moyamoya disease. Neurol Med Chir (Tokyo). 2005; 45:495–500.8. Kikuta K, Takagi Y, Nozaki K, Hanakawa T, Okada T, Mikuni N, et al. Asymptomatic microbleeds in moyamoya disease: T2*-weighted gradient-echo magnetic resonance imaging study. J Neurosurg. 2005; 102:470–475.
Article9. Kuroda S, Kashiwazaki D, Ishikawa T, Nakayama N, Houkin K. Incidence, locations, and longitudinal course of silent microbleeds in moyamoya disease: a prospective T2*-weighted MRI study. Stroke. 2013; 44:516–518.10. Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y. Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007; 38:1430–1435.
Article11. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis. Neurol Med Chir (Tokyo). 2012; 52:245–266.12. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997; 99:Suppl 2. S238–S240.13. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003; 34:1126–1129.
Article14. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008; 39:42–47.
Article15. Hoshino H, Izawa Y, Suzuki N. Research Committee on Moyamoya Disease. Epidemiological features of moyamoya disease in Japan. Neurol Med Chir (Tokyo). 2012; 52:295–298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of the Ivy Sign on Fluid-Attenuated Intensity Recovery Images in Improved Brain Hemodynamic Changes after Superficial Temporal Artery-Middle Cerebral Artery Anastomosis in Adult Patients with Moyamoya Disease
- Usefulness of Combined Fat- and Fluid-Suppressed SPIR-FLAIR Images in Optic Neuritis: Comparison with Fat-Suppressed SPIR or STIR Images or STIR images
- Importance of Contrast-Enhanced Fluid-Attenuated Inversion Recovery Magnetic Resonance Imaging in Various Intracranial Pathologic Conditions
- Reversible Cerebral Vasoconstriction Syndrome Presenting as Transient Vessel Wall Enhancement on Contrast-Enhanced Fluid-Attenuated Inversion Recovery Images: A Case Report and Literature Review
- Fast Fluid-Attenuated Inversion-Recovery MR Image in the Intracranial Tumors : Comparison with Fast Spin-echoImage