Ann Surg Treat Res.
2016 Apr;90(4):183-193. 10.4174/astr.2016.90.4.183.
Characterization of sphere-forming HCT116 clones by whole RNA sequencing
- Affiliations
-
- 1R&D Center, L&K Biomed Co. Ltd., Seoul, Korea.
- 2Department of Surgery, College of Medicine, Kyung Hee University, Seoul, Korea. issac34@korea.com
- KMID: 2160947
- DOI: http://doi.org/10.4174/astr.2016.90.4.183
Abstract
- PURPOSE
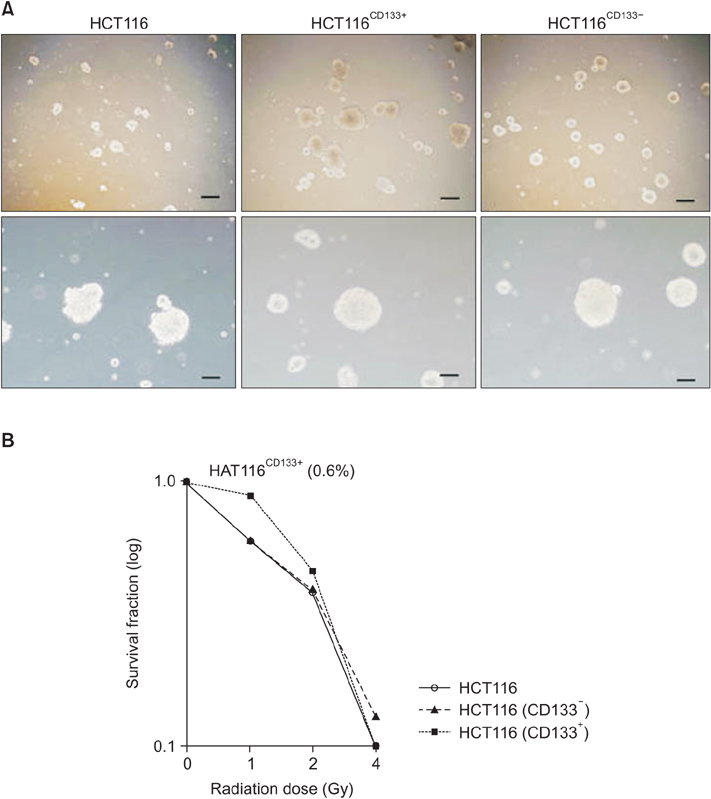
To determine CD133+ cells defined as cancer stem cells (CSCs) in colon cancer, we examined whether CD133+ clones in HCT116 demonstrate known features of CSCs like sphere-forming ability, chemodrug-resistance, and metastatic potential.
METHODS
Magnetic cell isolation and cell separation demonstrated that <1% of HCT116 cells expressed CD133, with the remaining cells being CD133- clones. In colon cancer cells, radioresistance is also considered a CSC characteristic. We performed clonogenic assay using 0.4 Gy γ-irradiation.
RESULTS
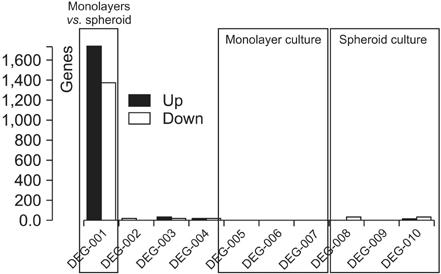
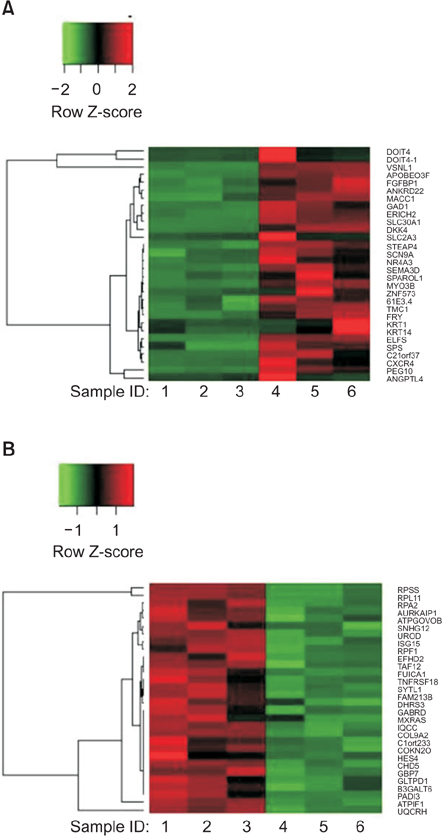
Interestingly, there were no differences between HCT116 parental and HCT116 CD133+ clones when the cells comprised 0.5% of the total cells, and CD133- clone demonstrated radiosensitive changes compared with parental and CD133+ clones. Comparing gene expression profiles between sphere-forming and nonforming culture conditions of HCT116 subclones by whole RNA sequencing failed to obtain specific genes expressed in CD133+ clones.
CONCLUSION
Despite no differences of gene expression profiles in monolayer attached culture conditions of each clone, sphere-forming conditions of whole HCT116 subclones, parental, CD133+, and CD133- increased 1,761 coding genes and downregulated 1,384 genes related to CSCs self-renewal and survival. Thus, spheroid cultures of HCT116 cells could be useful to expand colorectal CSCs rather than clonal expansion depending on CD133 expressions.
MeSH Terms
Figure
Reference
-
1. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014; 14:275–291.2. Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic . Cell Stem Cell. 2014; 15:692–705.3. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007; 445:106–110.4. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007; 445:111–115.5. Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007; 1:389–402.6. Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014; 14:342–356.7. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013; 501:328–337.8. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012; 125(Pt 23):5591–5596.9. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012; 21:309–322.10. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009; 9:239–252.11. Ren F, Sheng WQ, Du X. CD133: a cancer stem cells marker, is used in colorectal cancers. World J Gastroenterol. 2013; 19:2603–2611.12. Wang BB, Li ZJ, Zhang FF, Hou HT, Yu JK, Li F. Clinical significance of stem cell marker CD133 expression in colorectal cancer. Histol Histopathol. 2016; 31:299–306.13. Chen S, Song X, Chen Z, Li X, Li M, Liu H, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2013; 8:e56380.14. Chen KL, Pan F, Jiang H, Chen JF, Pei L, Xie FW, et al. Highly enriched CD133(+) CD44(+) stem-like cells with CD133(+)CD44(high) metastatic subset in HCT116 colon cancer cells. Clin Exp Metastasis. 2011; 28:751–763.15. Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006; 98:1755–1757.16. Bleau AM, Zandueta C, Redrado M, Martínez-Canarias S, Larzabal L, Montuenga LM, et al. Sphere-derived tumor cells exhibit impaired metastasis by a host-mediated quiescent phenotype. Oncotarget. 2015; 6:27288–27303.17. Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011; 10:1378–1384.18. Li Q, Shu Y. Role of solute carriers in response to anticancer drugs. Mol Cell Ther. 2014; 2:15.19. Lee DG, Lee JH, Choi BK, Kim MJ, Kim SM, Kim KS, et al. H+-myo-inositol transporter SLC2A13 as a potential marker for cancer stem cells in an oral squamous cell carcinoma. Curr Cancer Drug Targets. 2011; 11:966–975.20. David M, Naudin C, Letourneur M, Polrot M, Renoir JM, Lazar V, et al. Suppressor of cytokine signaling 1 modulates invasion and metastatic potential of colorectal cancer cells. Mol Oncol. 2014; 8:942–955.21. Asfaha S, Hayakawa Y, Muley A, Stokes S, Graham TA, Ericksen RE, et al. Krt19(+)/Lgr5(-) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell. 2015; 16:627–638.22. Trautmann F, Cojoc M, Kurth I, Melin N, Bouchez LC, Dubrovska A, et al. CXCR4 as biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014; 90:687–699.23. Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014; 17:261–274.24. Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007; 13:4042–4045.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Whole Transcriptome Sequencing Data: Workflow and Software
- YM155, specific survivin inhibitor, can enhance artesunate-induced cytotoxicity in HCT116 colon cancer cells
- Characterization of Dermatophagoides pteronyssinus-specific T cell clones from bronchial asthmatics
- Effects of β-carotene on Expression of Selected MicroRNAs, Histone Acetylation, and DNA Methylation in Colon Cancer Stem Cells
- Tumorsphere formation and cancer stem cell characterization of REM134 canine mammary carcinoma cells