Yonsei Med J.
2008 Oct;49(5):811-818. 10.3349/ymj.2008.49.5.811.
Imaging of Viral Thymidine Kinase Gene Expression by Replicating Oncolytic Adenovirus and Prediction of Therapeutic Efficacy
- Affiliations
-
- 1Division of Nuclear Medicine, Department of Radiology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. yunmijin@yuhs.ac
- 2Institute for Cancer Research, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2158200
- DOI: http://doi.org/10.3349/ymj.2008.49.5.811
Abstract
- PURPOSE
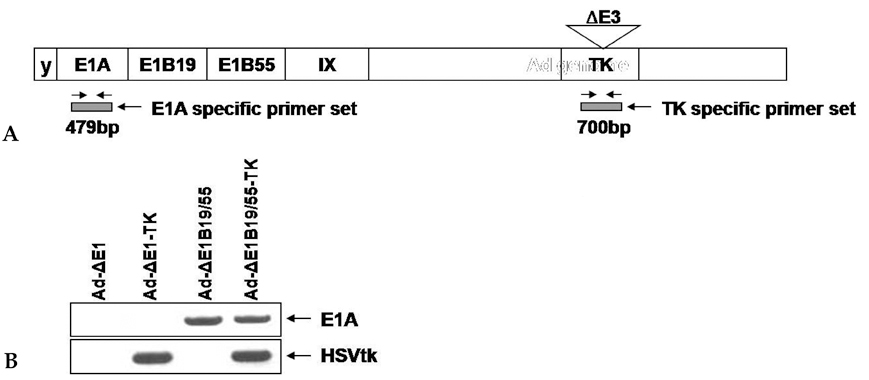
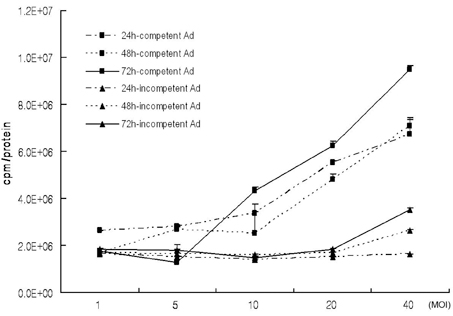
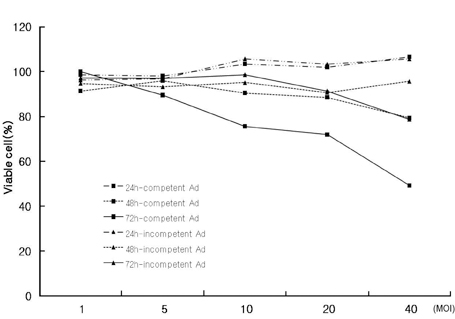
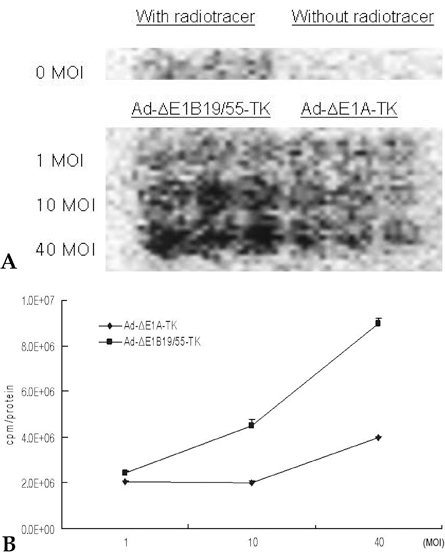
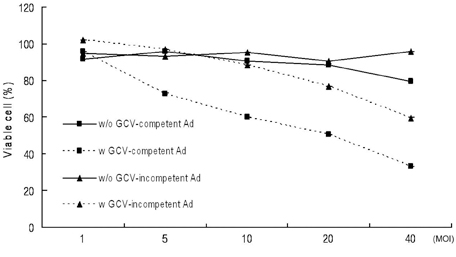
We have used a genetically attenuated adenoviral vector which expresses HSVtk to assess the possible additive role of suicidal gene therapy for enhanced oncolytic effect of the virus. Expression of TK was measured using a radiotracer-based molecular counting and imaging system. MATERIALS AND METHODS: Replication-competent recombinant adenoviral vector (Ad-deltaE1B19/55) was used in this study, whereas replication-incompetent adenovirus (Ad-deltaE1A) was generated as a control. Both Ad-deltaE1B19/55-TK and Ad-deltaE1A-TK comprise the HSVtk gene inserted into the E3 region of the viruses. YCC-2 cells were infected with the viruses and incubated with 2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl-5-iodouracil (I-131 FIAU) to measure amount of radioactivity. The cytotoxicity of the viruses was determined, and gamma ray imaging of HSVtk gene was performed. MTT assay was also performed after GCV treatment. RESULTS: On gamma counter-analyses, counts/minute (cpm)/microgram of protein showed MOIs dependency with deltaE1B19/55-TK infection. On MTT assay, Ad-deltaE1B19/55-TK led to more efficient cell killing than Ad-deltaE1A-TK. On plate imaging by gamma camera, both Ad-deltaE1B19/55-TK and Ad-deltaE1A-TK infected cells showed increased I-131 FIAU uptake in a MOI dependent pattern, and with GCV treatment, cell viability of deltaE1B19/55-TK infection was remarkably reduced compared to that of Ad-deltaE1A-TK infection. CONCLUSION: Replicating Ad-deltaE1B19/55-TK showed more efficient TK expression even in the presence of higher-cancer cell killing effects compared to non-replicating Ad-deltaE1A-TK. Therefore, GCV treatment still possessed an additive role to oncolytic effect of Ad-deltaE1B19/55-TK. The expression of TK by oncolytic viruses could rapidly be screened using a radiotracer-based counting and imaging technique.
Keyword
MeSH Terms
-
Adenoviridae/*genetics/physiology
Cell Line, Transformed
Cell Line, Tumor
Ganciclovir/pharmacology
Gene Expression
Gene Therapy/methods
Genetic Vectors
Humans
*Oncolytic Virotherapy
Oncolytic Viruses/*genetics/physiology
Simplexvirus/genetics
Tetrazolium Salts/analysis
Thiazoles/analysis
Thymidine Kinase/*genetics/metabolism
Transgenes
Viral Proteins/*genetics/metabolism
Virus Replication
Figure
Reference
-
1. Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004. 4:1–14.
Article2. Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002. 1575:1–14.
Article3. Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001. 7:33–40.
Article4. Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000. 105:1169–1172.
Article5. Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001. 8:89–98.
Article6. Wildner O, Morris JC, Vahanian NN, Ford H Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999. 6:57–62.
Article7. Raki M, Hakkarainen T, Bauerschmitz GJ, Särkioja M, Desmond RA, Kanerva A, et al. Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther. 2007. 14:1380–1388.
Article8. Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002. 9:725–736.
Article9. Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996. 274:373–376.
Article10. Lee H, Kim J, Lee B, Chang JW, Ahn J, Park JO, et al. Oncolytic potential of E1B 55 kDa-deleted YKL-1 recombinant adenovirus: correlation with p53 functional status. Int J Cancer. 2000. 88:454–463.
Article11. Dix BR, O'Carroll SJ, Myers CJ, Edwards SJ, Braithwaite AW. Efficient induction of cell death by adenoviruses requires binding of E1B55k and p53. Cancer Res. 2000. 60:2666–2672.12. Braithwaite AW, Russell IA. Induction of cell death by adenoviruses. Apoptosis. 2001. 6:359–370.13. Nanda D, Vogels R, Havenga M, Avezaat CJ, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001. 61:8743–8750.14. Haviv YS, Takayama K, Glasgow JN, Blackwell JL, Wang M, Lei X, et al. A model system for the design of armed replicating adenoviruses using p53 as a candidate transgene. Mol Cancer Ther. 2002. 1:321–328.15. Edholm D, Molin M, Bajak E, Akusjärvi G. Adenovirus vector designed for expression of toxic proteins. J Virol. 2001. 75:9579–9584.
Article16. Lambright ES, Amin K, Wiewrodt R, Force SD, Lanuti M, Propert KJ, et al. Inclusion of the herpes simplex thymidine kinase gene in a replicating adenovirus does not augment antitumor efficacy. Gene Ther. 2001. 8:946–953.
Article17. Zinn KR, Chaudhuri TR, Buchsbaum DJ, Mountz JM, Rogers BE. Simultaneous evaluation of dual gene transfer to adherent cells by gamma-ray imaging. Nucl Med Biol. 2001. 28:135–144.
Article18. Johnson M, Sato M, Burton J, Gambhir SS, Carey M, Wu L. Micro-PET/CT monitoring of herpes thymidine kinase suicide gene therapy in a prostate cancer xenograft: the advantage of a cell-specific transcriptional targeting approach. Mol Imaging. 2005. 4:463–472.19. Jacobs A, Voges J, Reszka R, Lercher M, Gossmann A, Kracht L, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001. 358:727–729.20. Cascante A, Abate-Daga D, Garcia-Rodríguez L, González JR, Alemany R, Fillat C. GCV modulates the antitumoural efficacy of a replicative adenovirus expressing the Tat8-TK as a late gene in a pancreatic tumour model. Gene Ther. 2007. 14:1471–1480.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Cytopathic Effect of Replicating Adenovirus Expressing Adenovirus Death Protein
- Enhanced Oncolytic Effect and Anti-Tumor Effect of Replication Competent Adenovirus with Double Mutation in E1A & E1B Regions
- Effects of Integrin avB5 and av B53 on the Adenovirus - Mediated Gene Transduction
- Tumor targeted gene therapy
- Adenovirus as a new agent for multiple myeloma therapies: Opportunities and restrictions