J Korean Med Sci.
2013 Jan;28(1):93-99. 10.3346/jkms.2013.28.1.93.
Computational Quantification of the Cardiac Energy Consumption during Intra-Aortic Balloon Pumping Using a Cardiac Electromechanics Model
- Affiliations
-
- 1Department of Medical IT Convergence Engineering, Kumoh National Institute of Technology, Gumi, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, & SMG-SNU Boramae Hospital, Seoul, Korea.
- 3Department of Mechanical and Biomedical Engineering, Kangwon National University, Chuncheon, Korea. ebshim@kangwon.ac.kr
- KMID: 2158007
- DOI: http://doi.org/10.3346/jkms.2013.28.1.93
Abstract
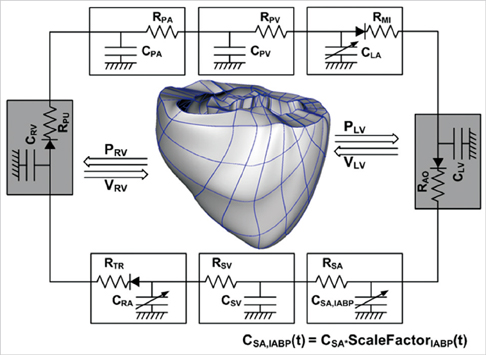
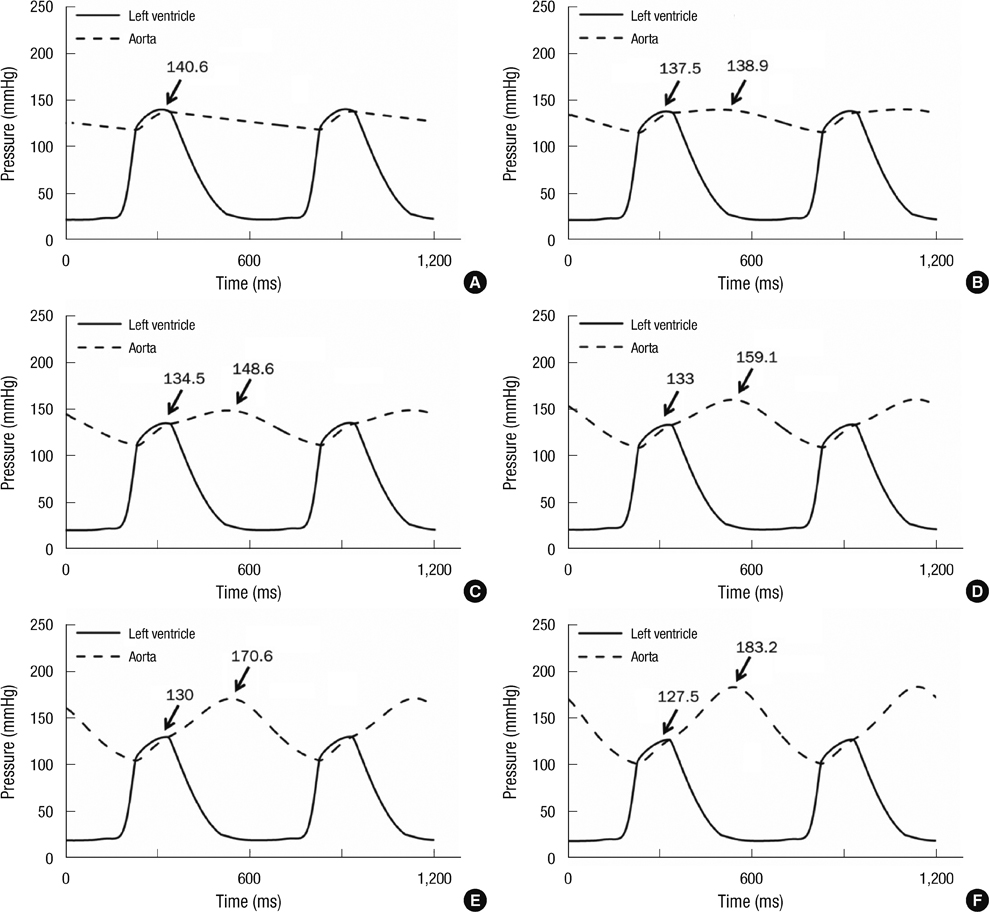
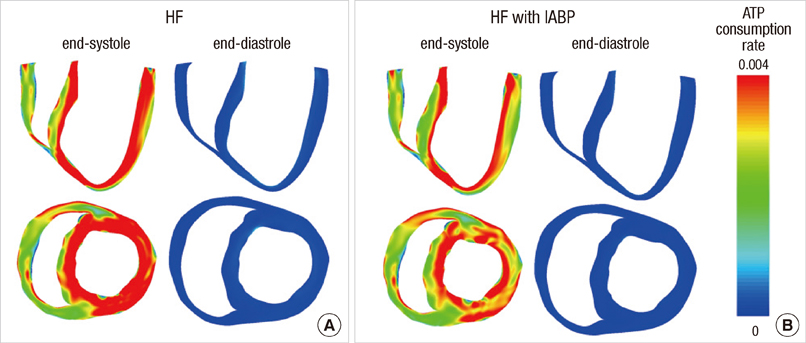
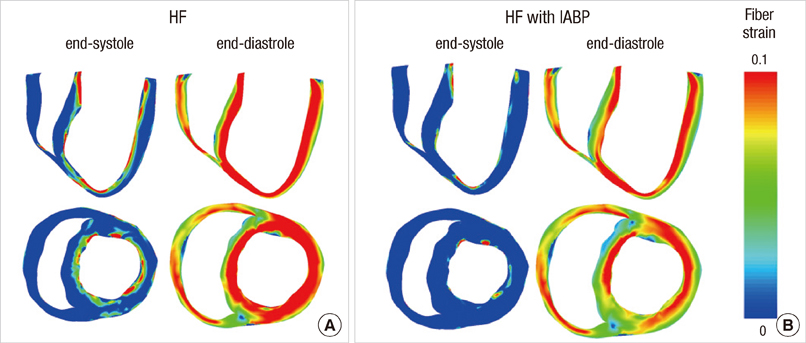
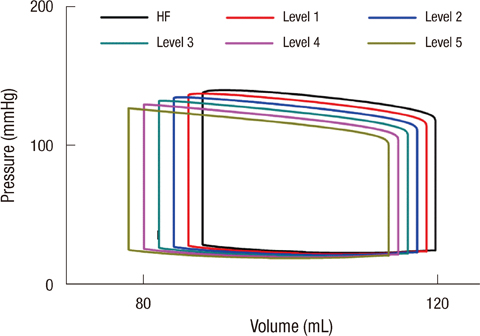
- To quantify the reduction in workload during intra-aortic balloon pump (IABP) therapy, indirect parameters are used, such as the mean arterial pressure during diastole, product of heart rate and peak systolic pressure, and pressure-volume area. Therefore, we investigated the cardiac energy consumption during IABP therapy using a cardiac electromechanics model. We incorporated an IABP function into a previously developed electromechanical model of the ventricle with a lumped model of the circulatory system and investigated the cardiac energy consumption at different IABP inflation volumes. When the IABP was used at inflation level 5, the cardiac output and stroke volume increased 11%, the ejection fraction increased 21%, the stroke work decreased 1%, the mean arterial pressure increased 10%, and the ATP consumption decreased 12%. These results show that although the ATP consumption is decreased significantly, stroke work is decreased only slightly, which indicates that the IABP helps the failed ventricle to pump blood efficiently.
MeSH Terms
Figure
Reference
-
1. Lin CY, Galysh FT, Ho KJ, Patel AS. Response to single-segment intraaortic balloon pumping as related to aortic compliance. Ann Thorac Surg. 1972. 13:468–476.2. Clark JW Jr, Kane GR, Bourland HM. On the feasibility of closed-loop control of intra-aortic balloon pumping. IEEE Trans Biomed Eng. 1973. 20:404–412.3. Barnea O, Moore TW, Dubin SE, Jaron D. Cardiac energy considerations during intraaortic balloon pumping. IEEE Trans Biomed Eng. 1990. 37:170–181.4. Suga H, Hisano R, Goto Y, Yamada O, Igarashi Y. Effect of positive inotropic agents on the relation between oxygen consumption and systolic pressure volume area in canine left ventricle. Circ Res. 1983. 53:306–318.5. Lim KM, Kim IS, Choi SW, Min BG, Won YS, Kim HY, Shim EB. Computational analysis of the effect of the type of LVAD flow on coronary perfusion and ventricular afterload. J Physiol Sci. 2009. 59:307–316.6. Lim KM, Constantino J, Gurev V, Zhu R, Shim EB, Trayanova NA. Comparison of the effects of continuous and pulsatile left ventricular-assist devices on ventricular unloading using a cardiac electromechanics model. J Physiol Sci. 2012. 62:11–19.7. Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008. 95:2368–2390.8. Gurev V, Lee T, Constantino J, Arevalo H, Trayanova NA. Models of cardiac electromechanics based on individual hearts imaging data: image-based electromechanical models of the heart. Biomech Model Mechanobiol. 2011. 10:295–306.9. Fox JJ, McHarg JL, Gilmour RF Jr. Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002. 282:H516–H530.10. Helm RH, Byrne M, Helm PA, Daya SK, Osman NF, Tunin R, Halperin HR, Berger RD, Kass DA, Lardo AC. Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation. 2007. 115:953–961.11. Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002. 106:1384–1389.12. O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999. 84:562–570.13. Dally S, Bredoux R, Corvazier E, Andersen JP, Clausen JD, Dode L, Fanchaouy M, Gelebart P, Monceau V, Del Monte F, et al. Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c). Biochem J. 2006. 395:249–258.14. Bassani JW, Bassani RA. SERCA upregulation: breaking the positive feedback in heart failure? Cardiovasc Res. 2005. 67:581–582.15. Muller-Ehmsen J, McDonough AA, Farley RA, Schwinger RH. Sodium pump isoform expression in heart failure: implication for treatment. Basic Res Cardiol. 2002. 97:Suppl 1. I25–I30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hyperthyroid Induced Cardiac Failure Requiring Intra-Aortic Balloon Pump during Thyroidectomy
- Cardiac Tamponade during Endovascular Repair of Thoracic Aortic Dissection
- Catastrophic Catecholamine-Induced Cardiomyopathy Mimicking Acute Myocardial Infarction, Rescued by Extracorporeal Membrane Oxygenation (ECMO) in Pheochromocytoma
- The Role of Intra-Aortic Balloon Pump in Coronary Artery Bypass Surgery
- Clinical Experience with IABP in Cardiac Surgery