J Korean Med Sci.
2012 Jul;27(7):777-783. 10.3346/jkms.2012.27.7.777.
Methionine Enhances the Contractile Activity of Human Colon Circular Smooth Muscle In Vitro
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kjparkmd@plaza.snu.ac.kr
- 2Department of Surgery, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- KMID: 2157923
- DOI: http://doi.org/10.3346/jkms.2012.27.7.777
Abstract
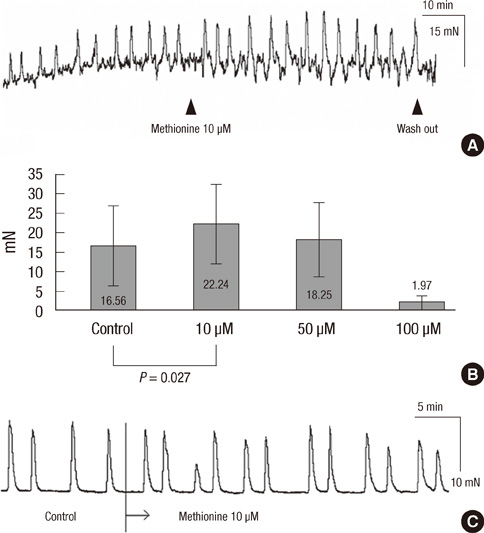
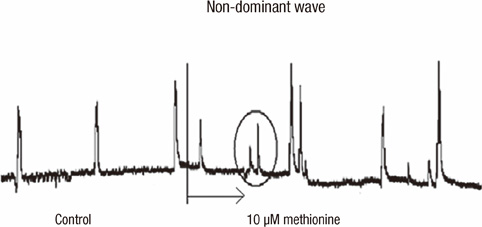
- Effective drug to manage constipation has been unsatisfactory. We sought to determine whether methionine has effect on the human colon. Human colon tissues were obtained from the specimens of colon resection. Microelectrode recording was performed and contractile activity of muscle strips and the propagation of the contractions in the colon segment were measured. At 10 microM, methionine depolarized the resting membrane potential (RMP) of circular muscle (CM) cells. In the CM strip, methionine increased the amplitude and area under the curve (AUC) of contractions. In the whole segment of colon, methionine increased the amplitude and AUC of the high amplitude contractions in the CM. These effects on contraction were maximal at 10 microM and were not observed in longitudinal muscles in both the strip and the colon segment. Methionine reversed the effects of pretreatment with sodium nitroprusside, tetrodotoxin and Nw-oxide-L-arginine, resulting in depolarization of the RMP, and increased amplitude and AUC of contractions in the muscle strip. Methionine treatment affected the wave pattern of the colon segment by evoking small sized amplitude contractions superimposed on preexisting wave patterns. Our results indicate that a compound mimicking methionine may provide prokinetic functions in the human colon.
Keyword
MeSH Terms
-
Area Under Curve
Arginine/pharmacology
Colon/drug effects/physiology
Humans
Membrane Potentials/drug effects
Methionine/*pharmacology
Microelectrodes
Muscle Contraction/*drug effects
Muscle, Smooth/drug effects/*physiology
Nitroprusside/pharmacology
Tetrodotoxin/pharmacology
Nitroprusside
Tetrodotoxin
Methionine
Arginine
Figure
Reference
-
1. Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005. 100:S5–S21.2. Longo WE, Vernava AM 3rd. Prokinetic agents for lower gastrointestinal motility disorders. Dis Colon Rectum. 1993. 36:696–708.3. Sanger GJ. Effects of metoclopramide and domperidone on cholinergically mediated contractions of human isolated stomach muscle. J Pharm Pharmacol. 1985. 37:661–664.4. Hasler WL. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007. 36:619–647.5. Prakash A, Wagstaff AJ. Domperidone. A review of its use in diabetic gastropathy. Drugs. 1998. 56:429–445.6. Wiley J, Owyang C. Dopaminergic modulation of rectosigmoid motility: action of domperidone. J Pharmacol Exp Ther. 1987. 242:548–551.7. Milo R. Use of the peripheral dopamine antagonist, domperidone, in the management of gastro-intestinal symptoms in patients with irritable bowel syndrome. Curr Med Res Opin. 1980. 6:577–584.8. Karamanolis G, Tack J. Promotility medications: now and in the future. Dig Dis. 2006. 24:297–307.9. Degen L, Matzinger D, Merz M, Appel-Dingemanse S, Osborne S, Lüchinger S, Bertold R, Maecke H, Beglinger C. Tegaserod, a 5-HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment Pharmacol Ther. 2001. 15:1745–1751.10. Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000. 118:463–468.11. Coremans G, Janssens J, Vantrappen G, Chaussade S, Ceccatelli P. Cisapride stimulates propulsive motility patterns in human jejunum. Dig Dis Sci. 1988. 33:1512–1519.12. Spiller R. Serotonergic modulating drugs for functional gastrointestinal diseases. Br J Clin Pharmacol. 2002. 54:11–20.13. Danielsson BR, Danielsson C, Nilsson MF. Embryonic cardiac arrhythmia and generation of reactive oxygen species: common teratogenic mechanism for IKr blocking drugs. Reprod Toxicol. 2007. 24:42–56.14. Schoenfeld P. Review article: the safety profile of tegaserod. Aliment Pharmacol Ther. 2004. 20:25–30.15. Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005. 144:1126–1137.16. Bright-Asare P, El-Bassoussi M. Cimetidine, metoclopramide, or placebo in the treatment of symptomatic gastroesophageal reflux. J Clin Gastroenterol. 1980. 2:149–156.17. McCallum RW. Review of the current status of prokinetic agents in gastroenterology. Am J Gastroenterol. 1985. 80:1008–1016.18. Bailey LD Jr, Stewart WR Jr, McCallum RW. New directions in the irritable bowel syndrome. Gastroenterol Clin North Am. 1991. 20:335–349.19. Reynolds JC. Prokinetic agents: a key in the future of gastroenterology. Gastroenterol Clin North Am. 1989. 18:437–457.20. Reynolds JC, Putnam PE. Prokinetic agents. Gastroenterol Clin North Am. 1992. 21:567–596.21. Lacy BE, Weiser K. Gastrointestinal motility disorders: an update. Dig Dis. 2006. 24:228–242.22. Herbst F, Kamm MA, Morris GP, Britton K, Woloszko J, Nicholls RJ. Gastrointestinal transit and prolonged ambulatory colonic motility in health and faecal incontinence. Gut. 1997. 41:381–389.23. Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001. 280:G629–G639.24. Kihara Y, Inoko M, Sasayama S. L-methionine augments mammalian myocardial contraction by sensitizing the myofilament to Ca2+. Circ Res. 1995. 77:80–87.25. Panagia V, Gupta MP, Ganguly PK, Dhalla NS. Methionine-induced positive inotropic effect in rat heart: possible role of phospholipid N-methylation. Circ Res. 1988. 62:51–55.26. Gupta MP, Panagia V, Dhalla NS. Phospholipid N-methylation-dependent alterations of cardiac contractile function by L-methionine. J Pharmacol Exp Ther. 1988. 245:664–672.27. Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992. 262:G379–G392.28. Ward SM, Dalziel HH, Thornbury KD, Westfall DP, Sanders KM. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992. 262:G237–G243.29. Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001. 533:155–163.30. Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, Horowitz B. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001. 276:44338–44346.31. Burrin DG, Stoll B. Emerging aspects of gut sulfur amino acid metabolism. Curr Opin Clin Nutr Metab Care. 2007. 10:63–68.32. Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004. 24:539–577.33. Hogeveen M, Blom HJ, Van Amerongen M, Boogmans B, Van Beynum IM, Van De Bor M. Hyperhomocysteinemia as risk factor for ischemic and hemorrhagic stroke in newborn infants. J Pediatr. 2002. 141:429–431.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanisms of Motility Change on Trinitrobenzenesulfonic Acid-Induced Colonic Inflammation in Mice
- Regional Differences in Intestinal Contractile Responses to Radial Stretch in the Human Lower Gastrointestinal Tract
- The Effects of Ketamine on Rat Myometrial Contractility
- The Effects of Nitroglycerin on the Contraction of Rat Uterine Smooth Muscle
- Neurotensin enhances gastric motility in antral circular muscle strip of guinea-pig.