J Korean Med Sci.
2011 Mar;26(3):346-351. 10.3346/jkms.2011.26.3.346.
Tumor Margin Histology Predicts Tumor Aggressiveness in Papillary Thyroid Carcinoma: A Study of 514 Consecutive Patients
- Affiliations
-
- 1Thyroid Cancer Center, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. surghsc@yuhs.ac
- 2Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2157870
- DOI: http://doi.org/10.3346/jkms.2011.26.3.346
Abstract
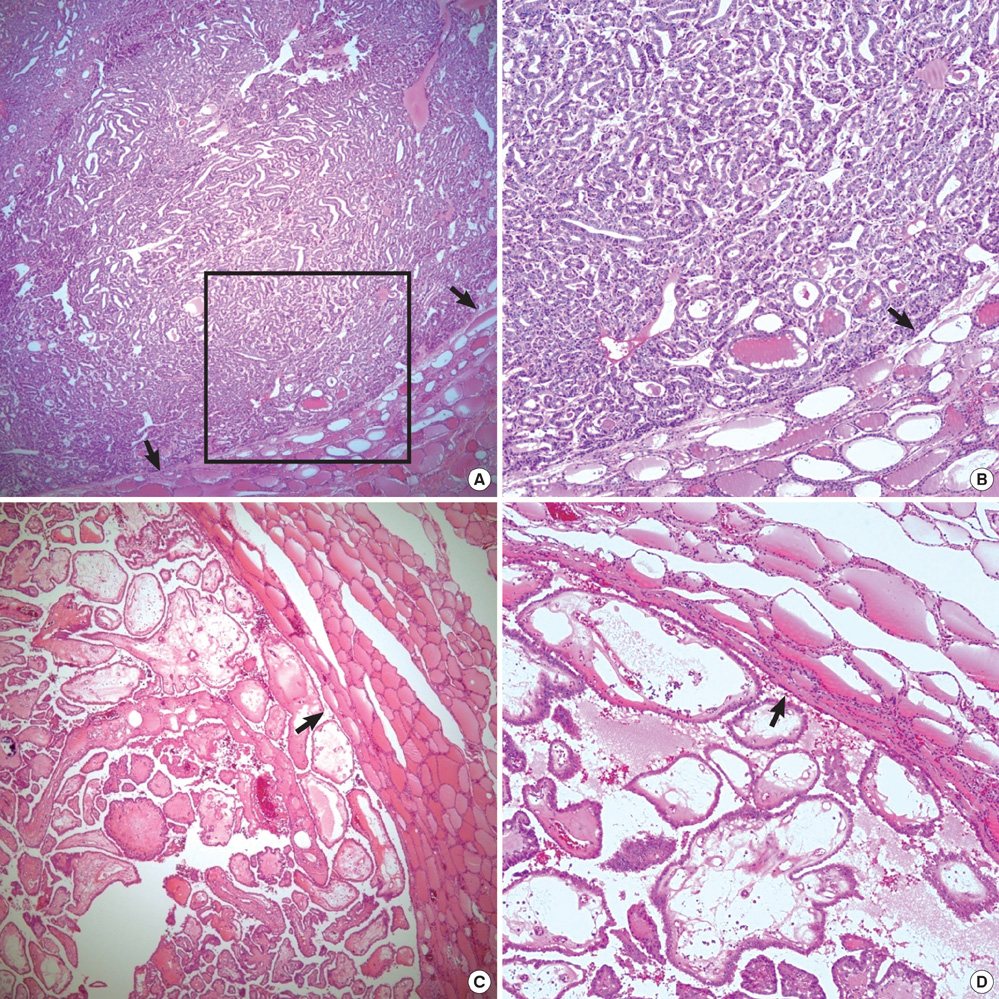
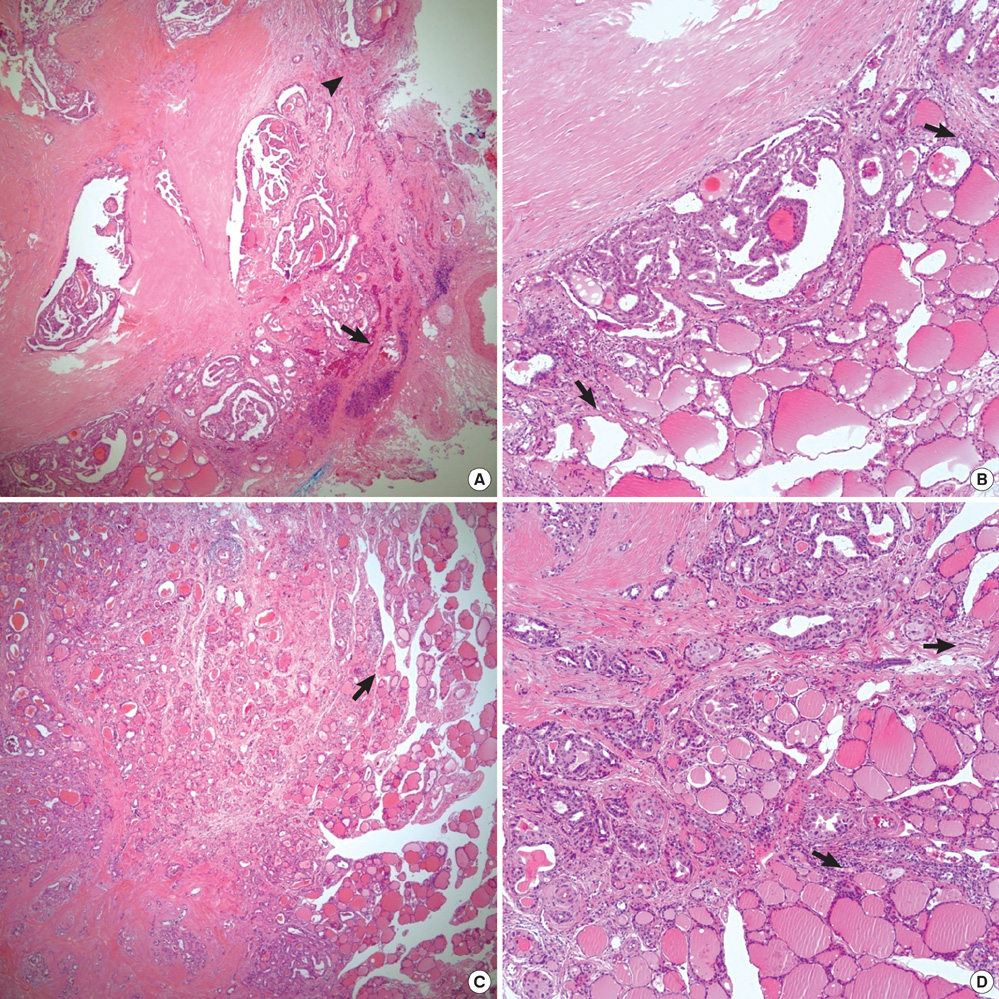
- Histologic patterns at tumor margins may be related to prognosis in several malignancies. We investigated tumor aggressiveness with respect to tumor margin histology in patients with papillary thyroid carcinoma (PTC). Five hundred fourteen consecutive patients who underwent surgery for primary PTC between January and July 2009 were assigned to two groups, one with an infiltrative pattern (I-type, n = 347) at tumor margins and one with an expanding pattern (E-type, n = 167). Tumor aggressiveness was assessed by analyzing relationships between these patterns and known prognostic factors. The analysis showed that unfavorable prognostic factors such as tumor multiplicity (P = 0.002), extrathyroidal extension (P < 0.001), lateral neck lymph node metastasis (P < 0.001) and advanced TNM stage (P = 0.001) were significantly more prevalent in patients with I-type PTC than in those with the E-type. Central neck node metastases were more prevalent without statistical significance in the I-type patients (P = 0.376). Tumor margin histology was not related to gender or tumor size. These results suggest that histologic patterns at tumor margins predict aggressiveness in PTC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.2. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981. 70:511–518.3. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990. 71:414–424.4. Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 2009. 7th ed. New York: Springer-Verlag.5. Lin YK, Sheng JM, Zhao WH, Wang WB, Yu XF, Teng LS, Ma ZM. Multifocal papillary thyroid carcinoma: clinical analysis of 168 cases. Zhonghua Wai Ke Za Zhi. 2009. 47:450–453.6. Kim ES, Kim TY, Koh JM, Kim YI, Hong SJ, Kim WB, Shong YK. Completion thyroidectomy in patients with thyroid cancer who initially underwent unilateral operation. Clin Endocrinol (Oxf). 2004. 61:145–148.7. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, Uchino S, Toda M, Sasaki A, Daa T, Nakayama I. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998. 8:197–202.8. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999. 84:458–463.9. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, Hong SJ, Gong G, Shong YK. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009. 71:581–586.10. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001. 25:632–637.11. Del Rio P, Cataldo S, Sommaruga L, Concione L, Arcuri MF, Sianesi M. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008. 33:1–5.12. Johnson TL, Lloyd RV, Thompson NW, Beierwaltes WH, Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1988. 12:22–27.13. Moreno Egea A, Rodriguez Gonzalez JM, Sola Perez J, Soria Cogollos T, Parrilla Paricio P. Prognostic value of the tall cell variety of papillary cancer of the thyroid. Eur J Surg Oncol. 1993. 19:517–521.14. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998. 82:740–753.15. Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: experience at a single institute. Pathology. 2008. 40:617–622.16. Caron NR, Clark OH. Well differentiated thyroid cancer. Scand J Surg. 2004. 93:261–271.17. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998. 338:297–306.18. Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987. 1:1303–1306.19. Rajaganeshan R, Prasad R, Guillou PJ, Chalmers CR, Scott N, Sarkar R, Poston G, Jayne DG. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer. 2007. 96:1112–1117.20. Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008. 14:3792–3797.21. González-Vela MC, Garijo MF, Fernández FA, Buelta L, Val-Bernal JF. Predictors of axillary lymph node metastases in patients with invasive breast carcinoma by a combination of classical and biological prognostic factors. Pathol Res Pract. 1999. 195:611–618.22. Barrio AV, Clark BD, Goldberg JI, Hoque LW, Bernik SF, Flynn LW, Susnik B, Giri D, Polo K, Patil S, Van Zee KJ. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007. 14:2961–2970.23. Mai KT, Perkins DG, Yazdi HM, Commons AS, Thomas J, Meban S. Infiltrating papillary thyroid carcinoma: review of 134 cases of papillary carcinoma. Arch Pathol Lab Med. 1998. 122:166–171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medullary and Papillary Thyroid Carcinoma as a Collision Tumor: Report of Five Cases
- Warthin-like Tumor Variant of Papillary Thyroid Carcinoma: A Case Report
- Granular Cell Tumor of Thyroid Gland That Was Concomitant with Papillary Thyroid Carcinoma: A Case Report
- Sonographic Findings of Thyroid Papillary Carcinoma
- Concurrent Medullay and Papillary Carcinoma of the Thyroid