Ann Dermatol.
2009 Nov;21(4):369-375. 10.5021/ad.2009.21.4.369.
The Inhibitory Effect of Phytoclear-EL1 on Melanogenesis
- Affiliations
-
- 1Department of Dermatology, Kosin University College of Medicine, Busan, Korea. ksderm98@unitel.co.kr
- KMID: 2156499
- DOI: http://doi.org/10.5021/ad.2009.21.4.369
Abstract
- BACKGROUND
Phytoclear-EL1, an extract from Euphorbia lathyris seeds, has a whitening effect due to inhibition of tyrosinase activity. OBJECTIVE: The purpose of this study was to investigate the inhibitory effect of phytoclear-EL1 on melanogenesis.
METHODS
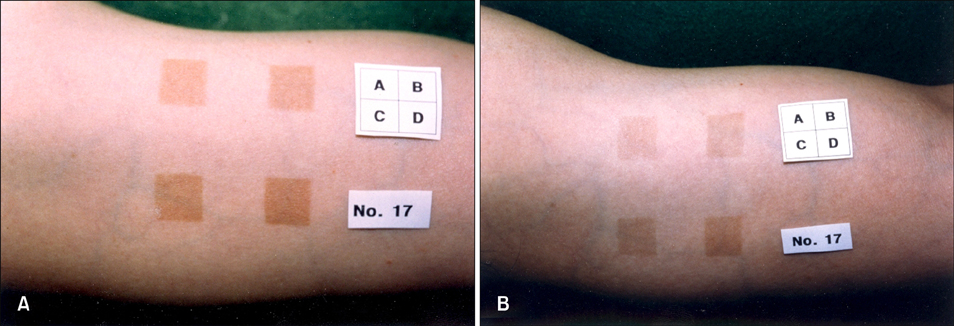
Cultured B-16 melanoma cells and 30 human volunteers were used for in vitro and in vivo studies, respectively. Phytoclear-EL1 was added to the cultured B-16 melanoma cells, and applied to UVB-induced hyperpigmented lesions of human volunteers twice daily for 7 weeks. Changes in the number of B-16 melanoma cells, as well as changes in morphology, melanin content, and tyrosinase activity, were measured and then compared with the normal control and the 10(-3)M arbutin groups. Also, the effect of phytoclear-EL1 on UVB-induced hyperpigmented lesions was examined through subjective and objective measurements.
RESULTS
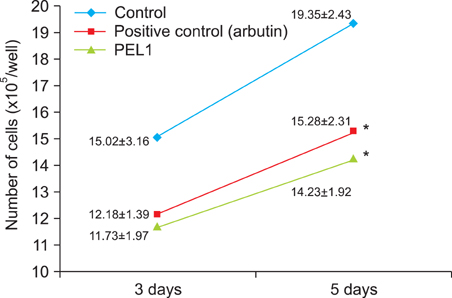
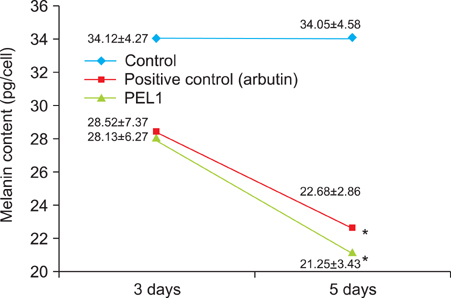
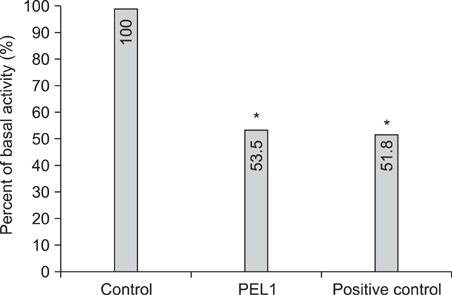
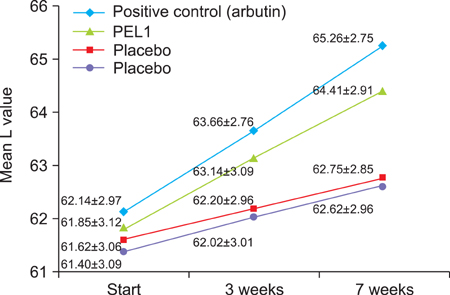
In the in vitro study (p<0.05), the number, melanin content, and tyrosinase activity of cultured B-16 melanoma cells were decreased in the 5microgram/ml phytoclear-EL1 group compared to the control group. On objective assessment with a chromameter, the 0.2% phytoclear-EL1 group had a larger difference in the mean L values before and 7 weeks after applying phytoclear-EL1 as compared to the other groups. On subjective assessment by both the researchers and subjects 7 weeks after applying experimental materials, the 0.2% phytoclear-EL1 group and positive control (3% arbutin) had higher scores than the placebo groups. These results demonstrated that phytoclear-EL1 in vivo and in vitro had an inhibitory effect on melanogenesis.
CONCLUSION
Phytoclear-EL1 may be a candidate extract in the control of hyperpigmentary disorders.
Keyword
MeSH Terms
Figure
Reference
-
1. Gendler EC. Topical treatment of the aging face. Dermatol Clin. 1997. 15:561–567.
Article2. Stratigos AJ, Katsambas AD. Optimal management of recalcitrant disorders of hyperpigmentation in dark-skinned patients. Am J Clin Dermatol. 2004. 5:161–168.
Article3. Masse MO, Duvallet V, Borremans M, Goeyens L. Identification and quantitative analysis of kojic acid and arbutine in skin-whitening cosmetics. Int J Cosmet Sci. 2001. 23:219–232.
Article4. Kim ST, Suh KS, Chae YS, Eom SC. The effect of arbutin, glycolic acid, kojic acid and pentadecenoic acid on the in vitro and in vivo pigmentary system after ultraviolet-B (UVB) irradiation. Korean J Dermatol. 1994. 32:977–989.5. Doh KS, Yoon JS, Park SG, Cho WG, Jang MS, Suh KS, et al. The effect of arbutin, medimin C and pinellia ternate extract on the pigmentary system. Korean J Invest Dermatol. 2001. 8:151–162.6. Andersson E, Rosdahl I, Torma H, Vahlquist A. Ultraviolet irradiation depletes cellular retinol and alters the metabolism of retinoic acid in cultured human keratinocytes and melanocytes. Melanoma Res. 1999. 9:339–346.
Article7. Cabanes J, Chazarra S, Garcia-Carmona F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J Pharm Pharmacol. 1994. 46:982–985.
Article8. Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006. 19:280–288.
Article9. Torok HM. A comprehensive review of the long-term and short-term treatment of melasma with a triple combination cream. Am J Clin Dermatol. 2006. 7:223–230.
Article10. Perez-Bernal A, Munoz-Perez MA, Camacho F. Management of facial hyperpigmentation. Am J Clin Dermatol. 2000. 1:261–268.
Article11. Kim CT, Jung MH, Kim HS, Kim HJ, Kang SJ, Kang SH. Inhibitors of melanogenesis from euphorbiae lathyridis semen. Korean J Pharmacognosy. 2000. 31:168–173.12. Masamoto Y, Ando H, Murata Y, Shimoishi Y, Tada M, Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem. 2003. 67:631–634.
Article13. Chang YH, Lee SH, Kang SJ. Novel whitening agent: Phytoclear-EL1. J Soc Cosmet Sci Korea. 2001. 27:111–118.14. Masamoto Y, Iida S, Kubo M. Inhibitory effect of Chinese crude drugs on tyrosinase. Planta Med. 1980. 40:361–365.
Article15. Gordon PR, Gilchrest BA. Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol. 1989. 93:700–702.
Article16. Pomerantz SH. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966. 241:161–168.
Article17. Halaban R, Hebert D, Fisher DE. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Biology of melanocytes. Fitzpatrick's dermatology in general medicine. 2003. 6th ed. New York: McGraw-Hill;127–148.18. Itokawa H, Ichihara Y, Watanabe K, Takeya K. An antitumor principle from Euphorbia lathyris. Planta Med. 1989. 55:271–272.19. Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000. 18:91–98.
Article20. Lemic-Stojcevic L, Nias AH, Breathnach AS. Effect of azelaic acid on melanoma cells in culture. Exp Dermatol. 1995. 4:79–81.
Article21. Hu F, Mah K, Teramura DJ. Effects of dicarboxylic acids on normal and malignant melanocytes in culture. Br J Dermatol. 1986. 114:17–26.
Article22. Boissy RE, Visscher M, DeLong MA. DeoxyArbutin: a novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp Dermatol. 2005. 14:601–608.
Article23. Assaf MH, Ali AA, Makboul MA, Beck JP, Anton R. Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Med. 1987. 53:343–345.
Article24. Mishima Y. A post melanosomal era: control of melanogenesis and melanoma growth. Pigment Cell Res. 1992. Suppl 2. 3–16.
Article25. Akiu S, Suzuki Y, Asahara T, Fujinuma Y, Fukuda M. Inhibitory effect of arbutin on melanogenesis--biochemical study using cultured B16 melanoma cells. Nippon Hifuka Gakkai Zasshi. 1991. 101:609–613.26. Suh KS, Roh HJ, Choi SY, Jeon YS, Doh KS, Bae JH, et al. Long-term evaluation of erythema and pigmentation induced by ultraviolet radiations of different wavelengths. Skin Res Technol. 2007. 13:154–161.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory and Eliminating Effects of Yeast-extracted Melanoston on Pigmentation and Preexisting Pigmentation, Respectively
- Phenolic Compounds from the Leaves of Stewartia pseudocamellia Maxim. and their Whitening Activities
- The Effects of SCH-T2 Seaweed Extract
- Whitening Effect of Cosmetics Containing Magnesium L-Ascorbyl-2-Phosphate(VC-PMG, Vitamin C Derivatives) Assessed by Colorimeter
- Tranexamic Acid Diminishes Laser-Induced Melanogenesis