Ann Dermatol.
2009 Nov;21(4):358-363. 10.5021/ad.2009.21.4.358.
The Clinical Features and Pathophysiology of Acute Radiation Dermatitis in Patients Receiving Tomotherapy
- Affiliations
-
- 1Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. drchosh@hotmail.com
- 2Department of Radiation Oncology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Clinical Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of General Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Hematology and Oncology of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2156497
- DOI: http://doi.org/10.5021/ad.2009.21.4.358
Abstract
- BACKGROUND
Radiation therapy (RT) including tomotherapy has been widely used to treat primary tumors, as well as to alleviate the symptoms of metastatic cancers.
OBJECTIVE
The primary purpose of this study was to examine the characteristics of the clinical features and pathophysiological mechanisms associated with acute radiation dermatitis in cancer patients that received tomotherapy, and compare the results to patients treated by conventional radiation therapy.
METHODS
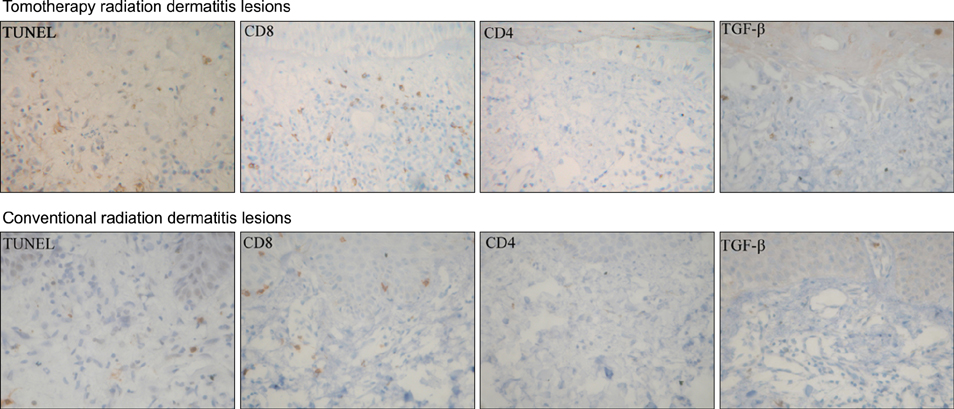
The study population consisted of 11 patients that were referred to the dermatology department because of radiation dermatitis after receiving tomotherapy; all patients were evaluated for clinical severity. The patients were assessed and identified using the National Cancer Institute Common Toxicity Criteria version (CTC) 3.0. We performed biopsies of the skin lesions that were examined for apoptosis using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labelling (TUNEL) assay and stained immunohistochemically with monoclonal antibodies to CD8, CD4 and TGF-beta. As a positive control, patients with radiation dermatitis treated with conventional radiation therapy were also studied.
RESULTS
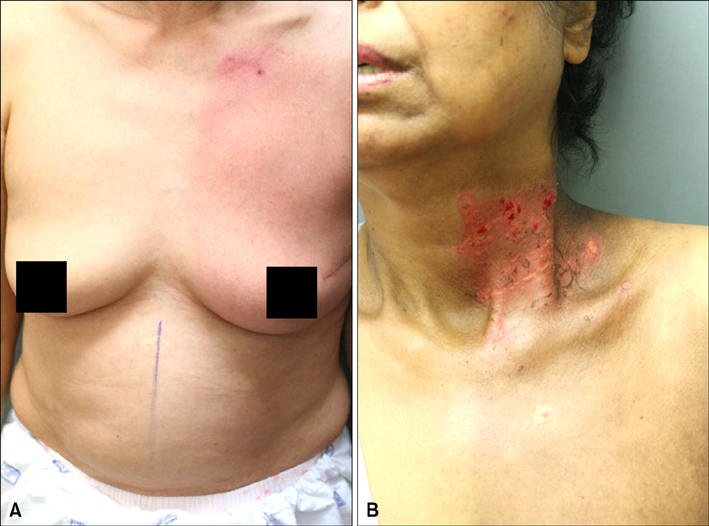
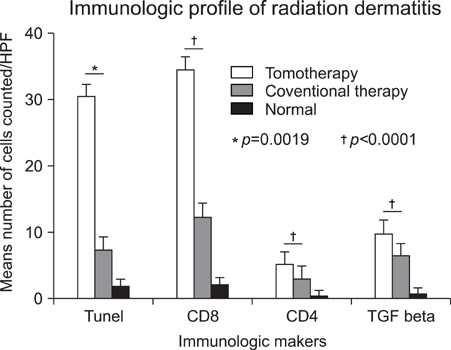
The results of the clinical features of the skin of tomotherapy patients were the following: grade 1 (36%), grade 2 (55%) and other changes (9%). Among the population that had skin lesions due to acute radiation dermatitis, the mean number of positive cells per high power field (HPF) was the following: there were 30.50+/-.50 TUNEL- positive cells, 34.60+/-12.50 CD8+ T cells, 5.19+/-3.17 CD4+T cells and 9.95+/-1.33 TGF-beta positive cells measured per HPF. The mean number of positive cells per HPF for the patients that received conventional radiation therapy was: TUNLEL-positive cells in 7.5+/-1.64, CD8-, CD4- and TGF-beta-positive cells in 12.50+/-3.73, 3.16+/- 1.47, 6.50+/-1.97.
CONCLUSION
We found that the number of TUNEL-positive cells and CD8+ T cells were higher in the lesions of patients receiving tomotherapy compared to the lesions of the patients receiving conventional radiation therapy. These findings suggest that tomotherapy without dose modification may cause significantly more severe forms of radiation dermatitis by apoptosis and cytotoxic immune responses than conventional radiation therapy.
MeSH Terms
Figure
Cited by 1 articles
-
Comment on "The Clinical Features and Pathophysiology of Acute Radiation Dermatitis in Patients Receiving Tomotherapy"
Slav Yartsev, Edward Yu
Ann Dermatol. 2011;23(Suppl 1):S139-S140. doi: 10.5021/ad.2011.23.S1.S139.
Reference
-
1. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006. 54:28–46.
Article2. Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001. 51:880–914.3. Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006. 29:66–70.
Article4. Frieben H. Demonstration eines Cancroid des rechten Handruckens, das sich nach langdauernder Einwirkung von Rontgenstrahlen entwickelt hatte. Fortschr Rontgenstr. 1902. 6:106–111.5. Hall EJ, Cox JD. Cox JD, Ang KK, editors. Physical and biological basis of radiation therapy. Radiation oncology. 2003. 8th ed. St. Louis: Mosby;3–62.6. Frederick DM, Renato GP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Radiobiology and radiation effects. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;858–862.7. Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000. 48:1307–1310.
Article8. Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2002. 15:216–224.
Article9. Peter RU, Beetz A, Ried C, Michel G, van Beuningen D, Ruzicka T. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat Res. 1993. 136:65–70.
Article10. James WD, Berger TG, Elston DM. Andrews' diseases of the skin: clinical dermatology. 2006. 10th ed. Philadelphia: Saunders Elsevier;39–41.11. Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother Oncol. 2002. 63:129–145.12. Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. J Invest Dermatol. 1981. 77:133–138.
Article13. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990. 57:751–773.
Article14. Mettler FA, Upton AC. Medical effects of ionizing radiation. 1995. 2nd ed. Philadelphia: W.B. Saunders;14–21.15. Canney PA, Dean S. Transforming growth factor beta: a promotor of late connective tissue injury following radiotherapy? Br J Radiol. 1990. 63:620–623.
Article16. Gruber BL, Marchese MJ, Kew RR. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994. 152:5860–5867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on "The Clinical Features and Pathophysiology of Acute Radiation Dermatitis in Patients Receiving Tomotherapy"
- Helical Tomotherapy: Image-guided Intensity Modulated Radiation Therapy
- Analysis on the Calculated Dose in the Lung Radiation Surgery Planning Using TomoTherpay
- Clinical Significance of Helical Tomotherapy in Children with Extracranial Disease
- Helical Tomotherapy in Elderly Prostate Cancer Patients