Korean J Pain.
2016 Jan;29(1):3-11. 10.3344/kjp.2016.29.1.3.
Neural Ablation and Regeneration in Pain Practice
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- KMID: 2151653
- DOI: http://doi.org/10.3344/kjp.2016.29.1.3
Abstract
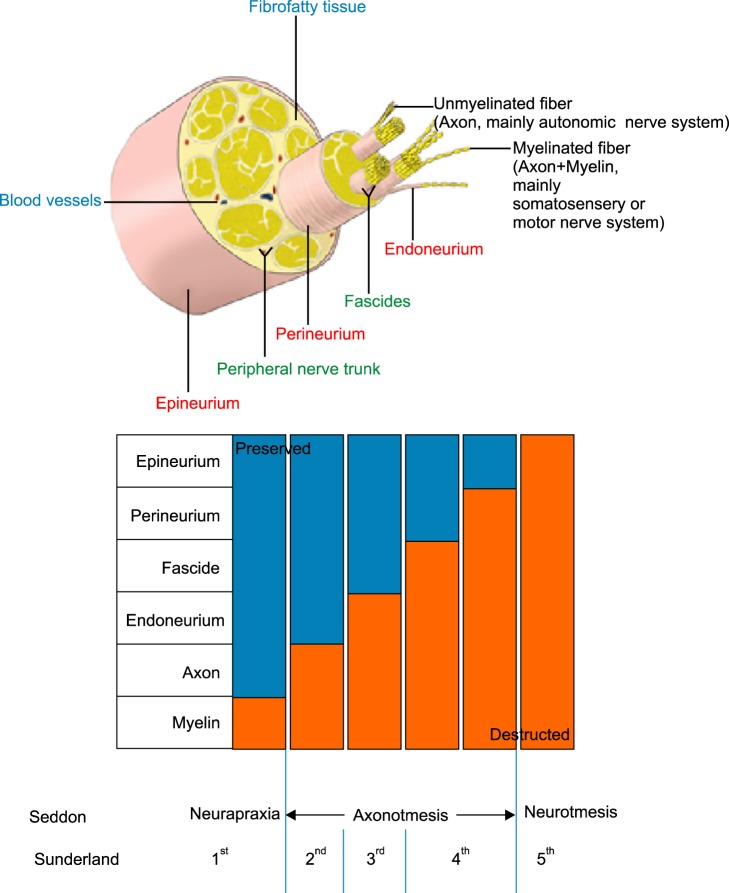
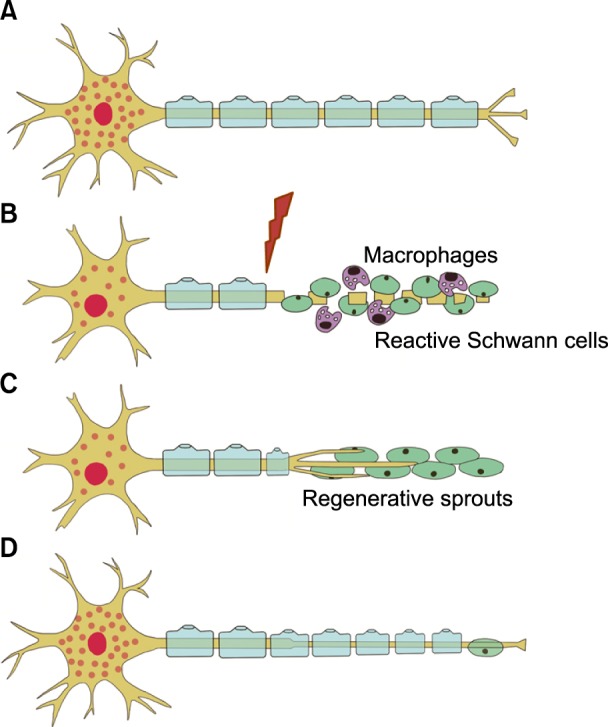
- A nerve block is an effective tool for diagnostic and therapeutic methods. If a diagnostic nerve block is successful for pain relief and the subsequent therapeutic nerve block is effective for only a limited duration, the next step that should be considered is a nerve ablation or modulation. The nerve ablation causes iatrogenic neural degeneration aiming only for sensory or sympathetic denervation without motor deficits. Nerve ablation produces the interruption of axonal continuity, degeneration of nerve fibers distal to the lesion (Wallerian degeneration), and the eventual death of axotomized neurons. The nerve ablation methods currently available for resection/removal of innervation are performed by either chemical or thermal ablation. Meanwhile, the nerve modulation method for interruption of innervation is performed using an electromagnetic field of pulsed radiofrequency. According to Sunderland's classification, it is first and foremost suggested that current neural ablations produce third degree peripheral nerve injury (PNI) to the myelin, axon, and endoneurium without any disruption of the fascicular arrangement, perineurium, and epineurium. The merit of Sunderland's third degree PNI is to produce a reversible injury. However, its shortcoming is the recurrence of pain and the necessity of repeated ablative procedures. The molecular mechanisms related to axonal regeneration after injury include cross-talk between axons and glial cells, neurotrophic factors, extracellular matrix molecules, and their receptors. It is essential to establish a safe, long-standing denervation method without any complications in future practices based on the mechanisms of nerve degeneration as well as following regeneration.
Keyword
MeSH Terms
-
Axons
Classification
Denervation
Electromagnetic Fields
Extracellular Matrix
Myelin Sheath
Nerve Block
Nerve Degeneration
Nerve Fibers
Nerve Growth Factors
Nerve Regeneration
Neuroglia
Neurons
Peripheral Nerve Injuries
Peripheral Nerves
Pulsed Radiofrequency Treatment
Recurrence
Regeneration*
Sympathectomy
Wallerian Degeneration
Nerve Growth Factors
Figure
Cited by 3 articles
-
Relationship between paravertebral muscle twitching and long-term effects of radiofrequency medial branch neurotomy
Jae Chul Koh, Do Hyeong Kim, Youn Woo Lee, Jong Bum Choi, Dong Hun Ha, Ji Won An
Korean J Pain. 2017;30(4):296-303. doi: 10.3344/kjp.2017.30.4.296.Lumbar burner and stinger syndrome in an elderly athlete
Veronika Wegener, Axel Stäbler, Volkmar Jansson, Christof Birkenmaier, Bernd Wegener
Korean J Pain. 2018;31(1):54-57. doi: 10.3344/kjp.2018.31.1.54.Ultrasound-guided pulsed radiofrequency ablation of stellate ganglion in upper-extremity phantom limb pain: a case series
Ajit Kumar, Manasa Kantha, Sonal Goyal, Pradeep Atter
Anesth Pain Med. 2024;19(4):349-352. doi: 10.17085/apm.24035.
Reference
-
1. Joo YC, Park JY, Kim KH. Comparison of alcohol ablation with repeated thermal radiofrequency ablation in medial branch neurotomy for the treatment of recurrent thoracolumbar facet joint pain. J Anesth. 2013; 27:390–395. PMID: 23192698.
Article2. Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007; 30:153–179. PMID: 17506644.
Article3. Ross LM, Lamperti ED. Arteries & veins of the rhoracic wall. In : Gilroy AM, MacPherson BR, Ross LM, editors. Atlas of anatomy. New York (NY): Thieme Medical Publishers, Ins;2005. p. 57.5. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951; 74:491–516. PMID: 14895767.
Article6. Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004; 16:E1. PMID: 15174821.
Article7. Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008; 119:1951–1965. PMID: 18482862.
Article8. Hsu M. Significance of clinical treatments on peripheral nerve and its effect on nerve regeneration. J Neurol Disord. 2014; 2:168.
Article9. Koyyalagunta D, Burton AW. The role of chemical neurolysis in cancer pain. Curr Pain Headache Rep. 2010; 14:261–267. PMID: 20524161.
Article10. American Society of Anesthesiologists Task Force on Chronic Pain Management. American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010; 112:810–833. PMID: 20124882.11. Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995; 80:290–295. PMID: 7818115.12. Iannuccilli JD, Prince EA, Soares GM. Interventional spine procedures for management of chronic low back pain-a primer. Semin Intervent Radiol. 2013; 30:307–317. PMID: 24436553.
Article13. Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block: efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010; 23:131–136. PMID: 20556215.
Article14. Kim WH, Kim SK, Lee CJ, Kim TH, Sim WS. Determination of adequate entry angle of lumbar sympathetic ganglion block in Korean. Korean J Pain. 2010; 23:11–17. PMID: 20552067.
Article15. Rocco AG, Palombi D, Raeke D. Anatomy of the lumbar sympathetic chain. Reg Anesth. 1995; 20:13–19. PMID: 7727322.16. Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014; 14:199–208. PMID: 24561446.
Article17. Erdine S, Bilir A, Cosman ER, Cosman ER Jr. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009; 9:407–417. PMID: 19761513.
Article18. Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien). 2011; 153:763–771. PMID: 21116663.
Article19. Rozen D, Parvez U. Pulsed radiofrequency of lumbar nerve roots for treatment of chronic inguinal herniorraphy pain. Pain Physician. 2006; 9:153–156. PMID: 16703977.20. Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg Clin N Am. 1991; 2:31–42. PMID: 1821734.
Article21. Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008; 12:37–41. PMID: 18417022.
Article22. Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997; 14:67–116. PMID: 9170101.
Article23. Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012; 98:16–37. PMID: 22609046.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Cord Regeneration in Rat using Neural Stem Cell Differentiated from Human Telencephalon
- Role of Glial Cells in Axonal Regeneration
- The Effect of Low Energy Laser Irradiation on the Sciatic Nerve Regeneration of the Rat
- Aucubin Promotes Neurite Outgrowth in Neural Stem Cells and Axonal Regeneration in Sciatic Nerves
- Large Circular Ring Catheter Ablation Versus Anatomically Guided Ablation of Atrial Fibrillation: Back to the Future for Successful Catheter Ablation of Atrial Fibrillation?