Immune Netw.
2012 Apr;12(2):66-69. 10.4110/in.2012.12.2.66.
Chemotherapeutic Candidate Inducing Immunological Death of Human Tumor Cell Lines
- Affiliations
-
- 1Office of Biomedical Professors, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. andyjosh@skku.edu
- 2College of Pharmacy & Division of Life and Pharmaceutical Sciences, Ewha Womans University, Seoul 120-750, Korea.
- 3Department of Microbiology & Immunology, Inje University College of Medicine, Busan 614-735, Korea.
- KMID: 2150736
- DOI: http://doi.org/10.4110/in.2012.12.2.66
Abstract
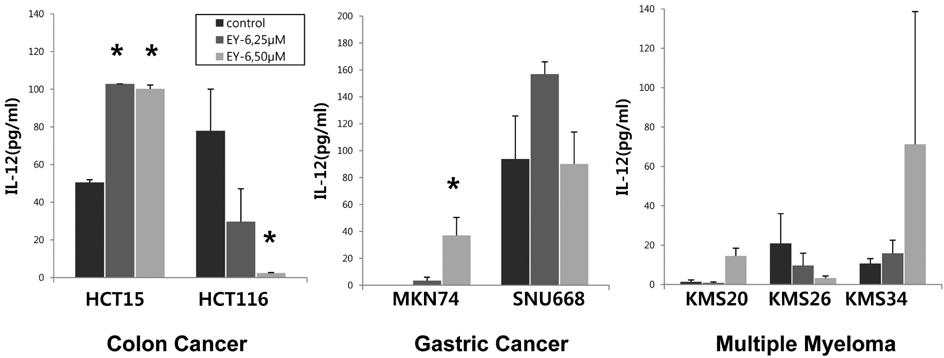
- The immunological death induction by EY-6 on the human tumor cell lines was screened. Human colon carcinoma (HCT15, HCT116), gastric carcinoma (MKN74, SNU668), and myeloma (KMS20, KMS26, KMS34) cells were died by EY-6 treatment with dose-dependent manner. CRT expression, a typical marker for the immunological death, was increased on the EY-6-treated colorectal and gastric cancer cells. Interestingly, the effects on the myeloma cell lines were complicated showing cell line dependent differential modulation. Cytokine secretion from the EY-6 treated tumor cells were dose and cell-dependent. IFN-gamma and IL-12 secretion was increased in the treated cells (200% to over 1000% of non-treated control), except HCT116, SNU668 and KMS26 cells which their secretion was declined by EY-6. Data suggest the potential of EY-6 as a new type of immuno-chemotherapeutics inducing tumor-specific cell death. Further studies are planned to confirm the efficacy of EY-6 including in vivo study.
MeSH Terms
Figure
Reference
-
1. Oh SJ, Ryu CK, Baek SY, Lee H. Cellular mechanism of newly synthesized indoledione derivative-induced immunological death of tumor cell. Immune Netw. 2011. 11:383–389.
Article2. Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from "silent" to immunogenic. Cancer Res. 2007. 67:7941–7944.
Article3. Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010. 16:3100–3104.
Article4. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009. 28:578–590.
Article5. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Métivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007. 13:54–61.
Article6. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005. 123:321–334.
Article7. Lee H, Baek S, Joe SJ, Pyo SN. Modulation of IFN-gamma production by TNF-alpha in macrophages from the tumor environment: significance as an angiogenic switch. Int Immunopharmacol. 2006. 6:71–78.
Article8. Esumi N, Hunt B, Itaya T, Frost P. Reduced tumorigenicity of murine tumor cells secreting gamma-interferon is due to nonspecific host responses and is unrelated to class I major histocompatibility complex expression. Cancer Res. 1991. 51:1185–1189.9. Janelidze S, Bexell D, Badn W, Darabi A, Smith KE, Fritzell S, Gunnarsson S, Milos P, Bengzon J, Salford LG, Siesjö P, Visse E. Immunizations with IFNgamma secreting tumor cells can eliminate fully established and invasive rat gliomas. J Immunother. 2009. 32:593–601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Effects of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)and its Molecular Mechanism in Human Gastric Cancer Cells
- Effect of TNF-alpha Gene Transfer to Respiratory Cancer Cell Lines onSensitivity to Anticancer drugs
- The Combination of TRAIL Treatment and Cancer Cell Selective Expression of TRAIL-Death Receptor DR4 Induces Cell Death in TRAIL-Resistant Cancer Cells
- Human hepatocellular carcinoma cells resist to TRAIL-induced apoptosis, and the resistance is abolished by cisplatin
- Expression of the FHIT gene Located in Chromosome 3p14.2 in Human Lung Cancer Cell Lines