Korean J Physiol Pharmacol.
2016 Jan;20(1):101-109. 10.4196/kjpp.2016.20.1.101.
Brief low [Mg2+]o-induced Ca2+ spikes inhibit subsequent prolonged exposure-induced excitotoxicity in cultured rat hippocampal neurons
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan 31116, Korea.
- 2Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. s-hyoon@catholic.ac.kr
- 3Catholic Neuroscience Institute, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2150479
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.101
Abstract
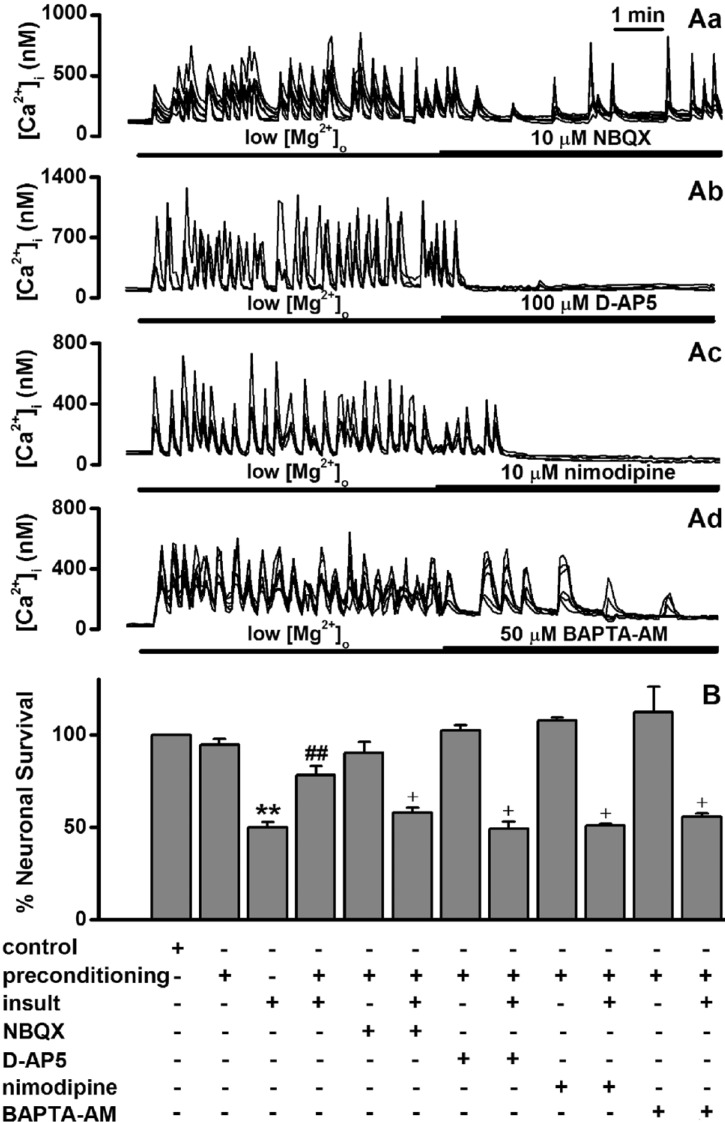
- Reducing [Mg2+]o to 0.1 mM can evoke repetitive [Ca2+]i spikes and seizure activity, which induces neuronal cell death in a process called excitotoxicity. We examined the issue of whether cultured rat hippocampal neurons preconditioned by a brief exposure to 0.1 mM [Mg2+]o are rendered resistant to excitotoxicity induced by a subsequent prolonged exposure and whether Ca2+ spikes are involved in this process. Preconditioning by an exposure to 0.1 mM [Mg2+]o for 5 min inhibited significantly subsequent 24 h exposure-induced cell death 24 h later (tolerance). Such tolerance was prevented by both the NMDA receptor antagonist D-AP5 and the L-type Ca2+ channel antagonist nimodipine, which blocked 0.1 mM [Mg2+]o-induced [Ca2+]i spikes. The AMPA receptor antagonist NBQX significantly inhibited both the tolerance and the [Ca2+]i spikes. The intracellular Ca2+ chelator BAPTA-AM significantly prevented the tolerance. The nonspecific PKC inhibitor staurosporin inhibited the tolerance without affecting the [Ca2+]i spikes. While Go6976, a specific inhibitor of PKCalpha had no effect on the tolerance, both the PKCepsilon translocation inhibitor and the PKCzeta pseudosubstrate inhibitor significantly inhibited the tolerance without affecting the [Ca2+]i spikes. Furthermore, JAK-2 inhibitor AG490, MAPK kinase inhibitor PD98059, and CaMKII inhibitor KN-62 inhibited the tolerance, but PI-3 kinase inhibitor LY294,002 did not. The protein synthesis inhibitor cycloheximide significantly inhibited the tolerance. Collectively, these results suggest that low [Mg2+]o preconditioning induced excitotoxic tolerance was directly or indirectly mediated through the [Ca2+]i spike-induced activation of PKCepsilon and PKCxi, JAK-2, MAPK kinase, CaMKII and the de novo synthesis of proteins.
MeSH Terms
-
Animals
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Cell Death
Cycloheximide
N-Methylaspartate
Neurons*
Nimodipine
Phosphatidylinositol 3-Kinases
Phosphotransferases
Rats*
Receptors, AMPA
Seizures
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Cycloheximide
N-Methylaspartate
Nimodipine
Phosphatidylinositol 3-Kinases
Phosphotransferases
Receptors, AMPA
Figure
Cited by 1 articles
-
Group 1 metabotropic glutamate receptor 5 is involved in synaptically-induced Ca2+-spikes and cell death in cultured rat hippocampal neurons
Ji Seon Yang, Sujeong Jeon, Hyun-Jong Jang, Shin Hee Yoon
Korean J Physiol Pharmacol. 2022;26(6):531-540. doi: 10.4196/kjpp.2022.26.6.531.
Reference
-
1. Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003; 34:325–337. PMID: 12909079.
Article2. Gurkoff GG, Shahlaie K, Lyeth BG. In vitro mechanical strain trauma alters neuronal calcium responses: Implications for posttraumatic epilepsy. Epilepsia. 2012; 53(Suppl 1):53–60.
Article3. Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987; 7:369–379. PMID: 2880938.
Article4. Allbritton NL, Oancea E, Kuhn MA, Meyer T. Source of nuclear calcium signals. Proc Natl Acad Sci U S A. 1994; 91:12458–12462. PMID: 7809059.
Article5. Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 1997; 16:7166–7173. PMID: 9384593.
Article6. Usachev YM, Thayer SA. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J Neurosci. 1997; 17:7404–7414. PMID: 9295386.7. Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998; 392:933–936. PMID: 9582075.
Article8. Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002; 277:27517–27527. PMID: 11971908.
Article9. Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005; 25:4279–4287. PMID: 15858054.10. Skaper SD, Facci L, Strijbos PJ. Neuronal protein kinase signaling cascades and excitotoxic cell death. Ann N Y Acad Sci. 2001; 939:11–22. PMID: 11462762.
Article11. Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci U S A. 2000; 97:436–441. PMID: 10618436.
Article12. Boeck CR, Ganzella M, Lottermann A, Vendite D. NMDA preconditioning protects against seizures and hippocampal neurotoxicity induced by quinolinic acid in mice. Epilepsia. 2004; 45:745–750. PMID: 15230696.
Article13. de Araújo Herculano B, Vandresen-Filho S, Martins WC, Boeck CR, Tasca CI. NMDA preconditioning protects against quinolinic acid-induced seizures via PKA, PI3K and MAPK/ERK signaling pathways. Behav Brain Res. 2011; 219:92–97. PMID: 21185872.
Article14. Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003; 23:384–391. PMID: 12533598.15. Bickler PE, Fahlman CS. Moderate increases in intracellular calcium activate neuroprotective signals in hippocampal neurons. Neuroscience. 2004; 127:673–683. PMID: 15283966.
Article16. Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990; 4:413–419. PMID: 1690567.
Article17. Rose K, Christine CW, Choi DW. Magnesium removal induces paroxysmal neuronal firing and NMDA receptor-mediated neuronal degeneration in cortical cultures. Neurosci Lett. 1990; 115:313–317. PMID: 1978266.
Article18. McLeod JR Jr, Shen M, Kim DJ, Thayer SA. Neurotoxicity mediated by aberrant patterns of synaptic activity between rat hippocampal neurons in culture. J Neurophysiol. 1998; 80:2688–2698. PMID: 9819273.
Article19. Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996; 16:4322–4334. PMID: 8699243.
Article20. Sombati S, Delorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol. 1995; 73:1706–1711. PMID: 7643176.
Article21. Kim HJ, Kim TH, Choi SJ, Hong YJ, Yang JS, Sung KW, Rhie DJ, Hahn SJ, Yoon SH. Fluoxetine suppresses synaptically induced [Ca2+]i spikes and excitotoxicity in cultured rat hippocampal neurons. Brain Res. 2013; 1490:23–34. PMID: 23131584.22. Deshpande LS, Lou JK, Mian A, Blair RE, Sombati S, Attkisson E, DeLorenzo RJ. Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: role of NMDA receptor activation and NMDA dependent calcium entry. Eur J Pharmacol. 2008; 583:73–83. PMID: 18289526.
Article23. Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987; 57:869–888. PMID: 3031235.
Article24. Velíšková J, Velíšek L. Gonadal status-dependent effects of in vivo β-estradiol administration to female rats on in vitro epileptiform activity induced by low [Mg2+]0 in combined hippocampusentorhinal cortex slices. Epilepsy Res. 2013; 107:297–301. PMID: 24113171.25. Dubinsky JM. Effects of calcium chelators on intracellular calcium and excitotoxicity. Neurosci Lett. 1993; 150:129–132. PMID: 8097028.
Article26. Jia J, Wang X, Li H, Han S, Zu P, Li J. Activations of nPKCepsilon and ERK1/2 were involved in oxygen-glucose deprivation-induced neuroprotection via NMDA receptors in hippocampal slices of mice. J Neurosurg Anesthesiol. 2007; 19:18–24. PMID: 17198096.27. Kim E, Raval AP, Defazio RA, Perez-Pinzon MA. Ischemic preconditioning via epsilon protein kinase C activation requires cyclooxygenase-2 activation in vitro. Neuroscience. 2007; 145:931–941. PMID: 17307294.
Article28. Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, Siddiqui MA, Das DK. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001; 33:1929–1936. PMID: 11708838.
Article29. Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol. 2008; 294:H257–H262. PMID: 17982005.
Article30. Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription1/3 pathway. Circulation. 2007; 116:535–544. PMID: 17606840.31. Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002; 22:10291–10301. PMID: 12451129.32. Tauskela JS, Brunette E, Monette R, Comas T, Morley P. Preconditioning of cortical neurons by oxygen-glucose deprivation: tolerance induction through abbreviated neurotoxic signaling. Am J Physiol Cell Physiol. 2003; 285:C899–C911. PMID: 12814913.33. Hatazaki S, Bellver-Estelles C, Jimenez-Mateos EM, Meller R, Bonner C, Murphy N, Matsushima S, Taki W, Prehn JH, Simon RP, Henshall DC. Microarray profile of seizure damage-refractory hippocampal CA3 in a mouse model of epileptic preconditioning. Neuroscience. 2007; 150:467–477. PMID: 17935890.
Article34. Tanaka K, Jimenez-Mateos EM, Matsushima S, Taki W, Henshall DC. Hippocampal damage after intra-amygdala kainic acid-induced status epilepticus and seizure preconditioning-mediated neuroprotection in SJL mice. Epilepsy Res. 2010; 88:151–161. PMID: 19931419.
Article35. Thompson SJ, Ashley MD, Stöhr S, Schindler C, Li M, McCarthy-Culpepper KA, Pearson AN, Xiong ZG, Simon RP, Henshall DC, Meller R. Suppression of TNF receptor-1 signaling in an in vitro model of epileptic tolerance. Int J Physiol Pathophysiol Pharmacol. 2011; 3:120–132. PMID: 21760970.36. Semenov DG, Samoilov MO, Łazarewicz JW. Calcium transients in the model of rapidly induced anoxic tolerance in rat cortical slices: involvement of NMDA receptors. Neurosignals. 2002; 11:329–335. PMID: 12566922.
Article37. Miyawaki H, Ashraf M. Ca2+ as a mediator of ischemic preconditioning. Circ Res. 1997; 80:790–799. PMID: 9168781.38. Tauskela JS, chakravarthy BR, Murray CL, Wang Y, Comas T, Hogan M, Hakim A, Morley P. Evidence from cultured rat cortical neurons of differences in the mechanism of ischemic preconditioning of brain and heart. Brain Res. 1999; 827:143–151. PMID: 10320703.
Article39. Matsumoto S, Shamloo M, Matsumoto E, Isshiki A, Wieloch T. Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II-alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2004; 24:54–61. PMID: 14688616.40. Vaccarino FM, Liljequist S, Tallman JF. Modulation of protein kinase C translocation by excitatory and inhibitory amino acids in primary cultures of neurons. J Neurochem. 1991; 57:391–396. PMID: 1649249.
Article41. Tejero-Díez P, Rodríguez-Sánchez P, Díez-Guerra FJ. Expression of protein kinase C isozymes in hippocampal neurones in culture. FEBS Lett. 1995; 363:293–298. PMID: 7737420.
Article42. Hajimohammadreza I, Probert AW, Coughenour LL, Borosky SA, Marcoux FW, Boxer PA, Wang KK. A specific inhibitor of calcium/calmodulin-dependent protein kinase-II provides neuroprotection against NMDA- and hypoxia/hypoglycemia-induced cell death. J Neurosci. 1995; 15:4093–4101. PMID: 7538570.
Article43. Waxham MN, Grotta JC, Silva AJ, Strong R, Aronowski J. Ischemiainduced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J Cereb Blood Flow Metab. 1996; 16:1–6. PMID: 8530541.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Apigenin on Glutamate-induced [Ca2+]i Increases in Cultured Rat Hippocampal Neurons
- Group 1 metabotropic glutamate receptor 5 is involved in synaptically-induced Ca2+ -spikes and cell death in cultured rat hippocampal neurons
- Magnesium suppresses the responses of dorsal horn cell to noxious stimuli in the rat
- Cyanidin-3-glucoside inhibits amyloid β₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons
- Low Non-NMDA Receptor Current Density as Possible Protection Mechanism from Neurotoxicity of Circulating Glutamate on Subfornical Organ Neurons in Rats