Cancer Res Treat.
2015 Jul;47(3):424-435. 10.4143/crt.2013.266.
Pemetrexed Continuation Maintenance in Patients with Nonsquamous Non-small Cell Lung Cancer: Review of Two East Asian Trials in Reference to PARAMOUNT
- Affiliations
-
- 1National Taiwan University Hospital, Taipei, Taiwan.
- 2Samsung Medical Center, Seoul, Korea.
- 3Kinki University Faculty of Medicine, Osaka, Japan.
- 4National Cancer Center Hospital, Tokyo, Japan.
- 5Eli Lilly Australia, West Ryde, Australia.
- 6Eli Lilly Japan, Kobe, Japan.
- 7Eli Lilly and Company, Taiwan/Hong Kong/Macao, China.
- 8Eli Lilly Interamerica, Buenos Aires, Argentina. mauro@lilly.com
- KMID: 2148491
- DOI: http://doi.org/10.4143/crt.2013.266
Abstract
- PURPOSE
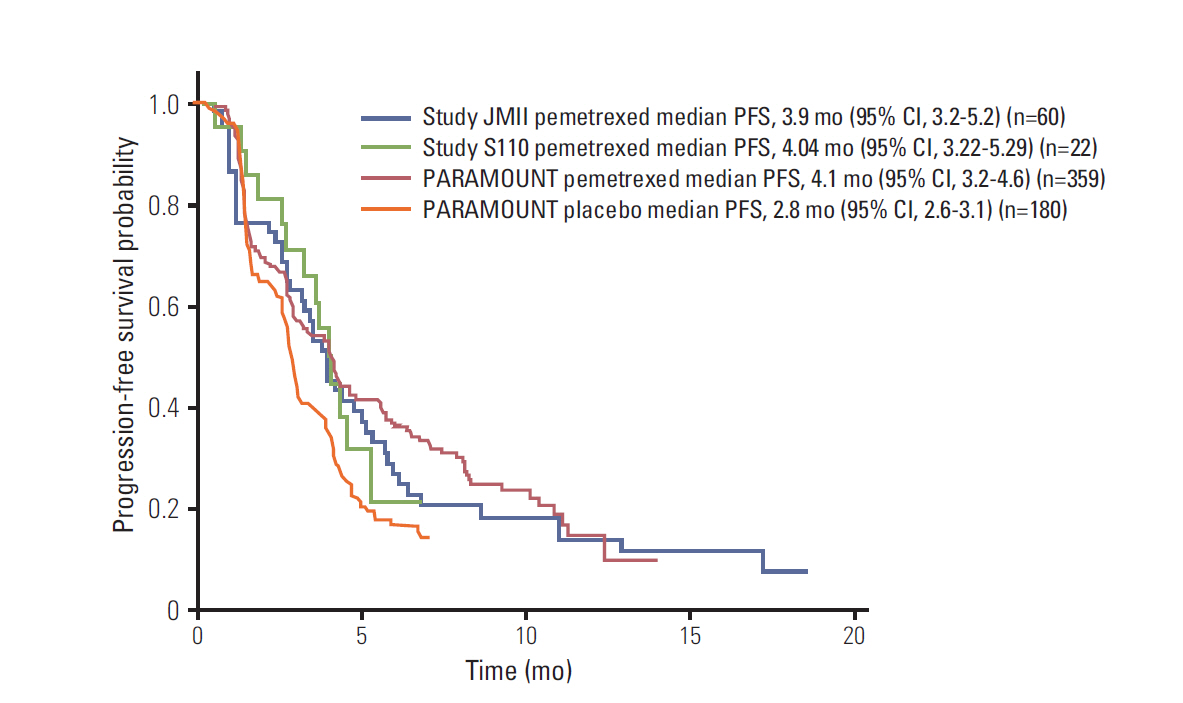
A recent phase III study (PARAMOUNT) demonstrated that pemetrexed continuation maintenance therapy is a new treatment paradigm for advanced nonsquamous non-small cell lung cancer (NSCLC). The majority of patients enrolled in PARAMOUNT were Caucasian (94%). We reviewed efficacy and safety data from two clinical trials, which enrolled East Asian (EA) patients, to supplement data from PARAMOUNT on pemetrexed continuation maintenance therapy in patients with nonsquamous NSCLC.
MATERIALS AND METHODS
Study S110 was a phase II, multicenter, randomized, controlled, open-label trial in never-smoker, chemonaive, EA patients (n=31) with locally advanced or metastatic nonsquamous NSCLC (n=27). Study JMII was a multicenter, open-label, single-arm, post-marketing, clinical trial in Japanese patients (n=109) with advanced nonsquamous NSCLC. PARAMOUNT was a multicenter, randomized, double-blind, placebo-controlled trial in patients with advanced nonsquamous NSCLC.
RESULTS
In EA patients with nonsquamous NSCLC, the median progression-free survival (PFS) for pemetrexed continuation maintenance therapy was 4.04 months (95% confidence interval [CI], 3.22 to 5.29 months) in study S110 and 3.9 months (95% CI, 3.2 to 5.2 months) in study JMII. The median PFS for pemetrexed continuation maintenance therapy in PARAMOUNT was 4.1 months (95% CI, 3.2 to 4.6 months). Pemetrexed continuation maintenance therapy in EA patients in studies S110 and JMII did not lead to any unexpected safety events, and was consistent with PARAMOUNT's safety profile.
CONCLUSION
The efficacy and safety data in the EA trials were similar to those in PARAMOUNT despite differences in patient populations and study designs. These data represent consistent evidence for pemetrexed continuation maintenance therapy in EA patients with advanced nonsquamous NSCLC.
MeSH Terms
Figure
Reference
-
References
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, version 2.2013 [Internet]. Fort Washington: National Comprehensive Cancer Network;2013. [cited 2013 Aug 22]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.2. Azzoli CG, Temin S, Giaccone G. 2011 Focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Oncol Pract. 2012; 8:63–6.
Article3. Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002; 20:1335–43.
Article4. Smith IE, O'Brien ME, Talbot DC, Nicolson MC, Mansi JL, Hickish TF, et al. Duration of chemotherapy in advanced nonsmall-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001; 19:1336–43.
Article5. Shih C, Chen VJ, Gossett LS, Gates SB, MacKellar WC, Habeck LL, et al. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997; 57:1116–23.6. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapynaive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.
Article7. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–97.
Article8. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for nonsmall-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–40.
Article9. Belani CP, Wu YL, Chen YM, Kim JH, Yang SH, Zhang L, et al. Efficacy and safety of pemetrexed maintenance therapy versus best supportive care in patients from East Asia with advanced, nonsquamous non-small cell lung cancer: an exploratory subgroup analysis of a global, randomized, phase 3 clinical trial. J Thorac Oncol. 2012; 7:567–73.
Article10. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012; 13:247–55.
Article11. Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013; 31:2895–902.
Article12. Ahn MJ, Yang JC, Liang J, Kang JH, Xiu Q, Chen YM, et al. Randomized phase II trial of first-line treatment with pemetrexed-cisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never-smoker patients with advanced non-small cell lung cancer. Lung Cancer. 2012; 77:346–52.
Article13. Okamoto I, Aoe K, Kato T, Hosomi Y, Yokoyama A, Imamura F, et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naive patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs. 2013; 31:1275–82.14. Gridelli C, de Marinis F, Pujol JL, Reck M, Ramlau R, Parente B, et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2012; 7:1713–21.
Article15. Mubarak N, Gaafar R, Shehata S, Hashem T, Abigeres D, Azim HA, et al. A randomized, phase 2 study comparing pemetrexed plus best supportive care versus best supportive care as maintenance therapy after first-line treatment with pemetrexed and cisplatin for advanced, non-squamous, non-small cell lung cancer. BMC Cancer. 2012; 12:423.
Article16. Karayama M, Inui N, Kuroishi S, Yokomura K, Toyoshima M, Shirai T, et al. Maintenance therapy with pemetrexed versus docetaxel after induction therapy with carboplatin and pemetrexed in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer: a randomized, phase II study. Cancer Chemother Pharmacol. 2013; 72:445–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pemetrexed-Erlotinib, Pemetrexed Alone, or Erlotinib Alone as Second-Line Treatment for East Asian and Non-East Asian Never-Smokers with Locally Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer: Exploratory Subgroup Analysis of a Phase II Trial
- Pemetrexed Continuation Maintenance versus Conventional Platinum-Based Doublet Chemotherapy in EGFR-Negative Lung Adenocarcinoma: Retrospective Analysis
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Interstitial Pneumonitis after Treatment with Pemetrexed for Non-small Cell Lung Cancer
- Epiphora following chemotherapy with pemetrexed in patients with advanced non-small cell lung cancer