Cancer Res Treat.
2015 Oct;47(4):616-629. 10.4143/crt.2014.051.
Pemetrexed-Erlotinib, Pemetrexed Alone, or Erlotinib Alone as Second-Line Treatment for East Asian and Non-East Asian Never-Smokers with Locally Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer: Exploratory Subgroup Analysis of a Phase II Trial
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, Seoul, Korea.
- 2Department of Thoracic Medical Oncology, Beijing Tumor Hospital and Institute, Beijing, China.
- 3Department of Respiratory Therapy, China Medical University and China Medical University Hospital, Taichung, Taiwan.
- 4Asia Pacific Statistical Sciences, Lilly China Drug Development and Medical Affairs Centre, Shanghai, China.
- 5Medical Department, Lilly Korea Ltd., Seoul, Korea.
- 6Medical Department, Eli-Lilly Interamerica Inc., Buenos Aires, Argentina. mauro@lilly.com
- KMID: 2403379
- DOI: http://doi.org/10.4143/crt.2014.051
Abstract
- PURPOSE
This subgroup analysis of a phase II trial was conducted to assess possible ethnicity-based trends in efficacy and safety in East Asian (EA) and non-EA populations with nonsquamous non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
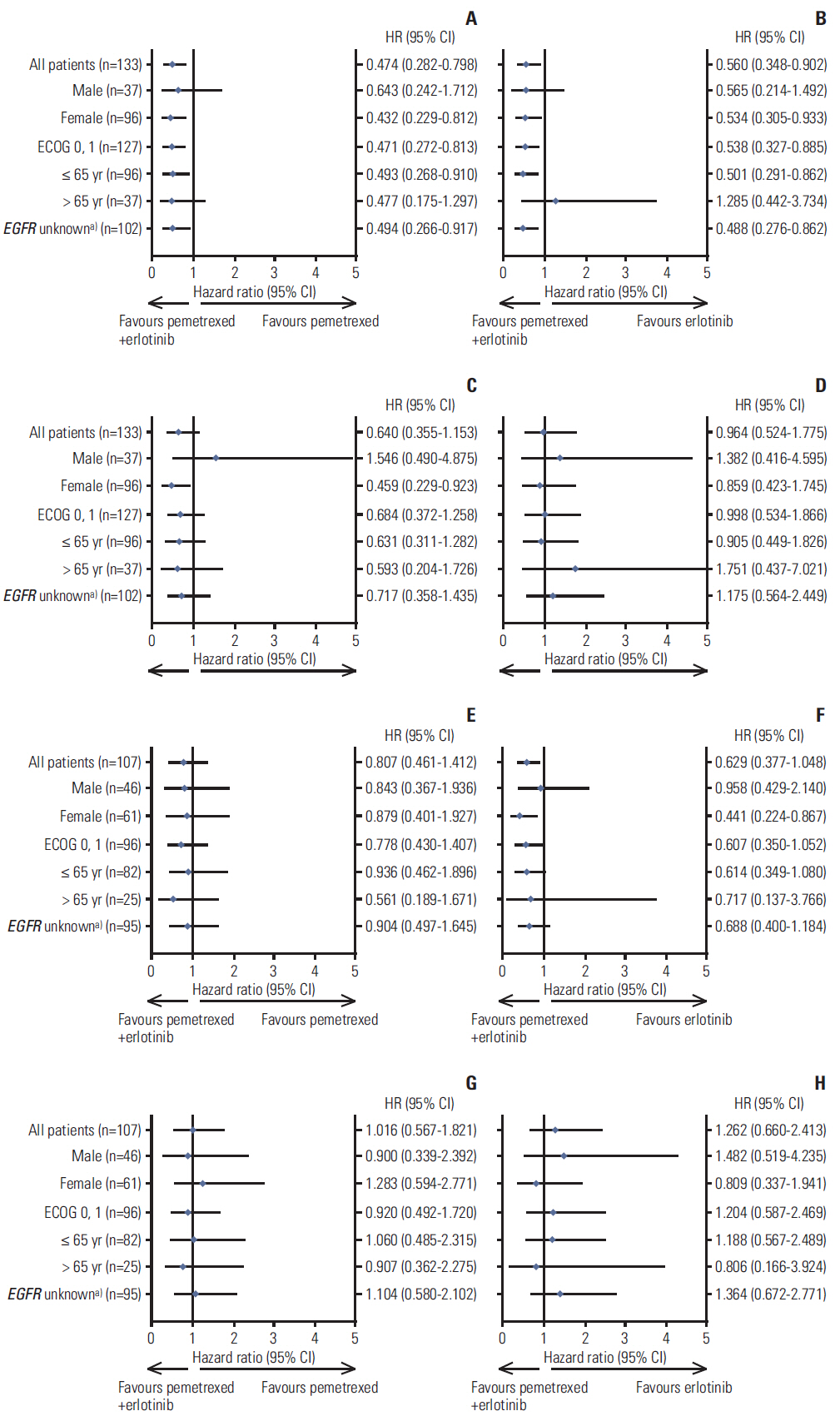
Never-smoker patients (n=240) with locally advanced or metastatic nonsquamous NSCLC included 133 EA patients randomized to pemetrexed supplemented with dexamethasone, folic acid, and vitamin B12 plus erlotinib (pemetrexed-erlotinib) (n=41), erlotinib (n=49), or pemetrexed (n=43), and 107 non-EA patients randomized to pemetrexed-erlotinib (n=37), erlotinib (n=33), or pemetrexed (n=37). The primary endpoint, progression-free survival (PFS), was analyzed using a multivariate Cox model.
RESULTS
Consistent with the results of the overall study, a statistically significant difference in PFS among the three arms was noted in the EA population favoring pemetrexed-erlotinib (overall p=0.003) as compared with either single-agent arm (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.29 to 0.79; p=0.004 vs. erlotinib; HR, 0.40; 95% CI, 0.23 to 0.70; p=0.001 vs. pemetrexed). The EA patients treated with pemetrexed-erlotinib achieved a longer median PFS (7.4 months) compared with erlotinib (4.5 months) and pemetrexed (4.0 months). The PFS results also numerically favored pemetrexed-erlotinib in the non-EA population (overall p=0.210) (HR, 0.62; 95% CI, 0.37 to 1.05; p=0.078 vs. erlotinib; HR, 0.75; 95% CI, 0.42 to 1.32; p=0.320 vs. pemetrexed) (median PFS: pemetrexed-erlotinib, 6.7 months; erlotinib, 3.0 months; pemetrexed, 4.4 months).
CONCLUSION
The PFS results from this subset analysis in both EA and non-EA populations are consistent with the results in the overall population. The PFS advantage for pemetrexed-erlotinib is significant compared with the single agents in EA patients.
MeSH Terms
Figure
Cited by 1 articles
-
Epidermal Growth Factor Receptor Mutation Status in the Treatment of Non-small Cell Lung Cancer: Lessons Learned
Dae Ho Lee, Vichien Srimuninnimit, Rebecca Cheng, Xin Wang, Mauro Orlando
Cancer Res Treat. 2015;47(4):549-554. doi: 10.4143/crt.2014.362.
Reference
-
References
1. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapynaive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–51.
Article2. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–40.
Article3. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–97.
Article4. Yang CH, Simms L, Park K, Lee JS, Scagliotti G, Orlando M. Efficacy and safety of cisplatin/pemetrexed versus cisplatin/gemcitabine as first-line treatment in East Asian patients with advanced non-small cell lung cancer: results of an exploratory subgroup analysis of a phase III trial. J Thorac Oncol. 2010; 5:688–95.
Article5. Sun Y, Wu YL, Zhou CC, Zhang L, Zhang L, Liu XY, et al. Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer. 2013; 79:143–50.
Article6. Ohe Y, Ichinose Y, Nakagawa K, Tamura T, Kubota K, Yamamoto N, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008; 14:4206–12.
Article7. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–32.
Article8. Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006; 24:3831–7.
Article9. Kaneda H, Yoshida T, Okamoto I. Molecularly targeted approaches herald a new era of non-small-cell lung cancer treatment. Cancer Manag Res. 2013; 5:91–101.10. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009; 361:958–67.
Article11. Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004; 64:8919–23.12. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–46.
Article13. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004; 22:777–84.
Article14. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial: INTACT 2. J Clin Oncol. 2004; 22:785–94.15. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005; 23:5892–9.
Article16. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007; 25:1545–52.
Article17. Lee DH, Lee JS, Kim SW, Rodrigues-Pereira J, Han B, Song XQ, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer. 2013; 49:3111–21.
Article18. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996; 17:343–6.
Article19. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article20. Von Pawel J, Papai-Szekely Z, Vinolas N, Sederholm C, Klima M, Desaiah D, et al. A randomized phase II study of pemetrexed versus pemetrexed plus erlotinib in second-line treatment for locally advanced or metastatic, nonsquamous NSCLC. J Clin Oncol. 2011; 29(S):Abstr 7526.
Article21. Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013; 14:777–86.
Article22. Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011; 29:4113–20.
Article23. Gahr S, Stoehr R, Geissinger E, Ficker JH, Brueckl WM, Gschwendtner A, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer. 2013; 109:1821–8.
Article24. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012; 30:1122–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pemetrexed Continuation Maintenance in Patients with Nonsquamous Non-small Cell Lung Cancer: Review of Two East Asian Trials in Reference to PARAMOUNT
- Clinical outcomes of erlotinib, gefitinib, or pemetrexed in patients with non-squamous, non-small-cell lung cancer
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
- Pemetrexed versus Gefitinib versus Erlotinib in Previously Treated Patients with Non-Small Cell Lung Cancer