J Korean Surg Soc.
2010 Oct;79(4):261-266. 10.4174/jkss.2010.79.4.261.
Sirolimus/steroids Maintenance Therapy after Early Cyclosporine Withdrawal: 12-month Efficacy and Safety Results of Multicenter Single Arm Pilot Study in Primary Renal Allograft Recipients in Korea
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. yukim@yuhs.ac
- 2Department of Surgery, Asan Medical Center, Seoul, Korea. djhan@amc.seoul.kr
- 3Department of Surgery, Catholic University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Kyungpook University College of Medicine, Daegu, Korea.
- 5Department of Internal Medicine, Keimyung University College of Medicine, Daegu, Korea.
- 6Department of Surgery, Samsung Medical Center, Seoul, Korea.
- 7Department of Surgery, Seoul National University Hospital, Seoul, Korea.
- 8Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 9Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2145027
- DOI: http://doi.org/10.4174/jkss.2010.79.4.261
Abstract
- PURPOSE
Sirolimus has potent anti-rejection activity as well as the ability to prolong allograft survival and reduce nephrotoxicity. This study was designed to evaluate the efficacy and safety of sirolimus in Korean de novo renal transplantation.
METHODS
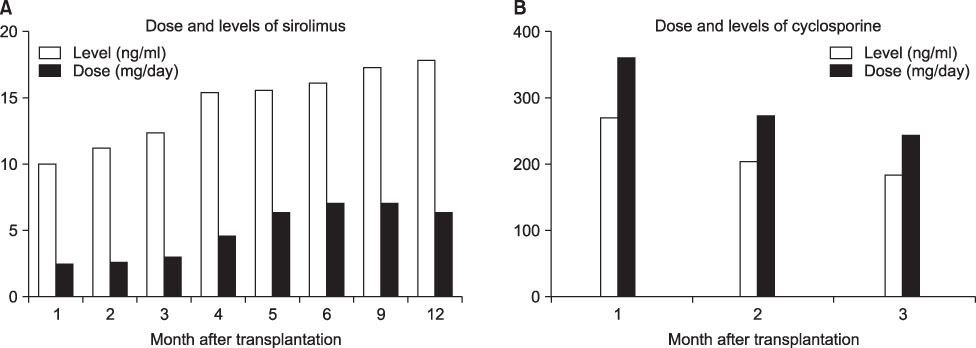
We included 79 patients who received sirolimus at nine Korean transplantation centers in the intention-to-treat and valid-for-safety analyses. The study was an open, single treatment arm multicenter trial with 12 months of patient follow-up. Initially, patients received 2 mg of sirolimus (after 6 mg of loading does) with cyclosporine and steroids. Sirolimus was administered for up to 12 months. Antibody induction was not used. At 3 months after transplantation, cyclosporine was progressively withdrawn over 4 to 8 weeks while sirolimus was adjusted to obtain trough concentrations within 15~30 ng/ml up to 6 months and concentrations within 12~24 ng/ml between 7 and 12 months.
RESULTS
The proportion of patients who completed the 12-month sirolimus medication per protocol was 74.7% (59/79). Cyclosporine withdrawal was possible in 64 recipients (81.0%). Fifteen patients discontinued sirolimus before cyclosporine withdrawal, and 5 recipients did so after successful cyclosporine withdrawal. Most common causes of sirolimus discontinuation were graft rejection (n=8). Incidence of biopsy-proven acute rejection within 6 months after transplantation was 15.2%. Patient and graft survival rates at 12 months post transplantation were 97.5% and 96.2%, respectively. During the study period, three graft losses occurred by patient death.
CONCLUSION
Based on this study, cyclosporine and sirolimus induction followed by cyclosporine withdrawal at 3 months post-transplant is considered to be efficient and safe after primary renal transplantation.
Keyword
MeSH Terms
Figure
Reference
-
1. Andoh TF, Burdmann EA, Bennett WM. Nephrotoxicity of immunosuppressive drugs: experimental and clinical observations. Semin Nephrol. 1997. 17:34–45.2. Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993. 24:472–495.3. Baeder WL, Adams LM. Effect of Rapamycin, Cyclosporin A and Prednisolone on Murine Thymocyte Proliferation In Vitro. 1989. Philadelphia: Wyeth-Ayerst Research;GTR-18812.4. Johnson RW, Kreis H, Oberbauer R, Brattström C, Claesson K, Eris J. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001. 72:777–786.5. Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation. 2003. 76:364–370.6. Stepkowski SM, Tian L, Napoli KL, Ghobrial R, Wang ME, Chou TC, et al. Synergistic mechanisms by which sirolimus and cyclosporin inhibit rat heart and kidney allograft rejection. Clin Exp Immunol. 1997. 108:63–68.7. Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Rapamune Study Group. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Transplantation. 1999. 68:1526–1532.8. Elashoff JD. nQuery Advisor Version 4.0 User's Guide. 2000. Los Angeles: Statistical Solution.9. Nankivell BJ, Gruenewald SM, Allen RD, Chapman JR. Predicting glomerular filtration rate after kidney transplantation. Transplantation. 1995. 59:1683–1689.10. Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus European Renal Transplant Study Group. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Transplantation. 1999. 67:1036–1042.11. Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000. 69:1252–1260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A 12-Month Single Arm Pilot Study to Evaluate the Efficacy and Safety of Sirolimus in Combination with Tacrolimus in Kidney Transplant Recipients at High Immunologic Risk
- A case of hemorrhagic fever with renal syndrome after renal transplantation
- Proliferation Signal Inhibitors: Sirolimus and Everolimus
- Safety and Efficacy of the Early Introduction of Everolimus (Certican(R)) with Low Dose of Cyclosporine in de Novo Kidney Recipients after 1 Month of Transplantation (Preliminary Results)
- Sirolimus-induced pneumonitis in a renal transplant recipient