J Korean Med Sci.
2015 Jun;30(6):682-687. 10.3346/jkms.2015.30.6.682.
A 12-Month Single Arm Pilot Study to Evaluate the Efficacy and Safety of Sirolimus in Combination with Tacrolimus in Kidney Transplant Recipients at High Immunologic Risk
- Affiliations
-
- 1The Research Institute for Transplantation, Yonsei University College of Medicine, Seoul, Korea. yukim@yuhs.ac
- 2Department of Transplantation Surgery, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- 3Department of Surgery, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 4Division of Nephrology, Department of Internal Medicine, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- 5Department of Laboratory Medicine, Severance Hospital, Yonsei University Health System, Seoul, Korea.
- KMID: 2160593
- DOI: http://doi.org/10.3346/jkms.2015.30.6.682
Abstract
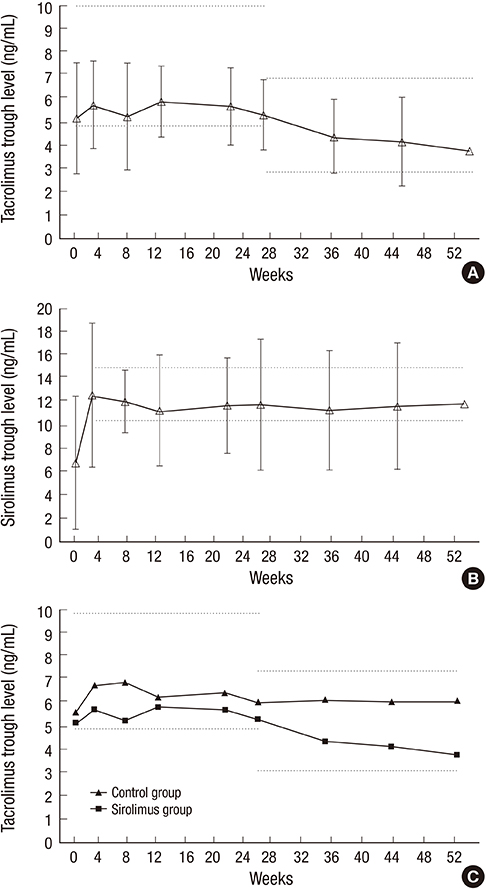
- The optimal immunosuppressive strategy for renal transplant recipients at high immunologic risk remains a topic of investigation. This prospective single arm pilot study was undertaken to evaluate the safety and efficacy of a combined tacrolimus and sirolimus regimen in recipients at immunological high risk and to compare outcomes with a contemporaneous control group received tacrolimus and mycophenolate mofetil. Patients that received a renal allograft between 2010 and 2011 at high risk (defined as panel reactive antibodies > 50%, 4 or more human leukocyte antigen mismatches, or retransplantation) were enrolled. All patients received basiliximab induction and corticosteroids. A total of 28 recipients treated with tacrolimus and sirolimus were enrolled in this study and 69 recipients were retrospectively reviewed as a control group. The sirolimus group showed a higher, but not statistically significant, incidence of biopsy proven acute rejection and a lower glomerular filtration rate than the control group. Furthermore, sirolimus group was associated with significant increases in BKV infection (P = 0.031), dyslipidemia (P = 0.004), and lymphocele (P = 0.020). The study was terminated prematurely due to a high incidence of adverse events. A de novo tacrolimus/sirolimus combination regimen may not be an ideal choice for recipients at high immunological risk.
Keyword
MeSH Terms
-
Adult
Drug Therapy, Combination/methods
Female
Graft Rejection/diagnosis/*etiology/*prevention & control
Humans
Immunocompromised Host
Immunosuppressive Agents/administration & dosage/adverse effects
Kidney Transplantation/*adverse effects
Longitudinal Studies
Male
Middle Aged
Sirolimus/*administration & dosage/adverse effects
Survival Rate
Tacrolimus/*administration & dosage/adverse effects
Treatment Outcome
Immunosuppressive Agents
Sirolimus
Tacrolimus
Figure
Cited by 1 articles
-
Sirolimus Combination with Tacrolimus in Kidney Transplant Recipients at High Immunological Risk: Observational Results 3 Years after Transplantation
Juhan Lee, Seung Hwan Song, Jae Geun Lee, Beom Seok Kim, Kyu Ha Huh, Yu Seun Kim
J Korean Soc Transplant. 2016;30(4):165-171. doi: 10.4285/jkstn.2016.30.4.165.
Reference
-
1. Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA. Relative importance of HLA mismatch and donor age to graft survival in young kidney transplant recipients. Transplantation. 2013; 96:469–475.2. Borobia AM, Romero I, Jimenez C, Gil F, Ramirez E, De Gracia R, Escuin F, Gonzalez E, Sansuán AJ. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009; 31:436–442.3. O'Seaghdha CM, McQuillan R, Moran AM, Lavin P, Dorman A, O'Kelly P, Mohan DM, Little P, Hickey DP, Conlon PJ. Higher tacrolimus trough levels on days 2-5 post-renal transplant are associated with reduced rates of acute rejection. Clin Transplant. 2009; 23:462–468.4. Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006; 6:514–522.5. Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011; 11:450–462.6. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004; 351:2715–2729.7. Vu MD, Qi S, Xu D, Wu J, Fitzsimmons WE, Sehgal SN, Dumont L, Busque S, Daloze P, Chen H. Tacrolimus (FK506) and sirolimus (rapamycin) in combination are not antagonistic but produce extended graft survival in cardiac transplantation in the rat. Transplantation. 1997; 64:1853–1856.8. van Hooff JP, Squifflet JP, Wlodarczyk Z, Vanrenterghem Y, Paczek L. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal-transplant recipients. Transplantation. 2003; 75:1934–1939.9. Vitko S, Wlodarczyk Z, Kyllönen L, Czajkowski Z, Margreiter R, Backman L, Perner F, Rigotti P, Jaques B, Abramowicz D, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant. 2006; 6:531–538.10. Gaber AO, Kahan BD, Van Buren C, Schulman SL, Scarola J, Neylan JF. Sirolimus High-Risk Study Group. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation. 2008; 86:1187–1195.11. Lo A, Egidi MF, Gaber LW, Shokouh-Amiri MH, Nazakatgoo N, Fisher JS, Gaber AO. Observations regarding the use of sirolimus and tacrolimus in high-risk cadaveric renal transplantation. Clin Transplant. 2004; 18:53–61.12. Shihab F, Christians U, Smith L, Wellen JR, Kaplan B. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014; 31:22–32.13. Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S. Prograf Study Group. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation. 2005; 80:303–309.14. Sampaio EL, Pinheiro-Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, Moreira S, Franco MF, Tedesco-Silva H Jr, Medina-Pestana JO. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen. Clin Transplant. 2008; 22:141–149.15. Lebranchu Y, Baan C, Biancone L, Legendre C, Morales JM, Naesens M, Thomusch O, Friend P. Pretransplant identification of acute rejection risk following kidney transplantation. Transpl Int. 2014; 27:129–138.16. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006; 355:1967–1977.17. Pilch NA, Taber DJ, Moussa O, Thomas B, Denmark S, Meadows HB, McGillicuddy JW, Srinivas TR, Baliga PK, Chavin KD. Prospective randomized controlled trial of rabbit antithymocyte globulin compared with IL-2 receptor antagonist induction therapy in kidney transplantation. Ann Surg. 2014; 259:888–893.18. Peddi VR, Wiseman A, Chavin K, Slakey D. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando). 2013; 27:97–107.19. Murakami N, Riella LV, Funakoshi T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta-analysis. Am J Transplant. 2014; 14:2317–2327.20. Lee HS, Huh KH, Kim YS, Kim MS, Kim HJ, Kim SI, Joo DJ. Sirolimus-induced pneumonitis after renal transplantation: a single-center experience. Transplant Proc. 2012; 44:161–163.21. Patel N, Taber DJ, Weimert NA, Fleming JN, Egidi FM, McGillicuddy J, Bratton CF, Lin A, Chavin KD, Baliga PK. Potential differences in kidney allograft outcomes between ethnicities when converting to sirolimus base immunosuppression. Transplant Proc. 2009; 41:4131–4137.22. Nashan B, Gaston R, Emery V, Säemann MD, Mueller NJ, Couzi L, Dantal J, Shihab F, Mulgaonkar S, Seun Kim Y, et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation. 2012; 93:1075–1085.23. Andrassy J, Hoffmann VS, Rentsch M, Stangl M, Habicht A, Meiser B, Fischereder M, Jauch KW, Guba M. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor-based immunosuppression? a systematic review and meta-analysis. Transplantation. 2012; 94:1208–1217.24. Grim SA, Pham T, Thielke J, Sankary H, Oberholzer J, Benedetti E, Clark NM. Infectious complications associated with the use of rituximab for ABO-incompatible and positive cross-match renal transplant recipients. Clin Transplant. 2007; 21:628–632.25. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007; 357:2601–2614.26. Langer RM, Hené R, Vitko S, Christiaans M, Tedesco-Silva H Jr, Ciechanowski K, Cassuto E, Rostaing L, Vilatoba M, Machein U, et al. Everolimus plus early tacrolimus minimization: a phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl Int. 2012; 25:592–602.27. Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, Wang Z, Zibari GB, Shihab F, Kim YS. Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation. 2013; 95:933–942.28. Shihab FS, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, Zibari G, Pattison J, Cornu-Artis C, Wang Z, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving reduced cyclosporine. Clin Transplant. 2013; 27:217–226.29. Dunn TB, Noreen H, Gillingham K, Maurer D, Ozturk OG, Pruett TL, Bray RA, Gebel HM, Matas AJ. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011; 11:2132–2143.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sirolimus Combination with Tacrolimus in Kidney Transplant Recipients at High Immunological Risk: Observational Results 3 Years after Transplantation
- A noninferiority, randomized controlled trial of late conversion to once-daily regimen of sirolimus and extended-release tacrolimus versus mycophenolic acid and extended-release tacrolimus for kidney transplant recipients
- A Pilot Study of Calcineurin Inhibitors (CNIs) and Steroid Avoidance Immunosuppressive Protocol among Living Donor Kidney Transplant Recipients
- Impact of tacrolimus intra-patient variability on kidney transplant outcomes according to immunologic risk
- Sirolimus/steroids Maintenance Therapy after Early Cyclosporine Withdrawal: 12-month Efficacy and Safety Results of Multicenter Single Arm Pilot Study in Primary Renal Allograft Recipients in Korea